September 2019
WWW.COPPERHOUNDPICTURES.COM
Our readers, authors, and advertisers often are surprised to discover that the magazine you hold in your hands (advertisements aside) represents primarily the work of three people. The BPI editors are multitaskers and jacks-of-all-trades by necessity. Every month we all seek out authors and work with them to develop their manuscripts . . . copyedit their texts and typeset the results . . . research, interview, and write our own articles . . . adapt and develop graphics . . . create layouts and page designs of both editorial and advertorial materials. . . acquire, track, and work with photography . . . and proofread each other’s results all on constantly rotating and overlapping deadlines. Add the need for a working familiarity of our subject matter and a thorough understanding of the English language — and you can imagine the sort of person our work requires.
You also might be surprised to know how little turnover this editorial staff has experienced over the years. Editor in c...
Photo Credit: www.stock.adobe.com
Serum and other blood-derived products have been used widely in pharmaceutical research for many years. Use of these materials has contributed to many different advances in human and veterinary health, and they continue to have an important role in drug development. Fetal bovine serum (FBS) has had a specific role in the culture of mammalian cells for over 60 years. It is proven to be a useful tool for a broad spectrum of applications because it supports a large range of mammalian cell types.
Directive 2010/63/EU covers the protection of animals used for scientific purposes, implementing the principles of the “3Rs” (replacement, reduction, and refinement) (
1
). It is a critical document on the use of animals and animal-derived materials in the scientific community working toward developing new products and methodologies to minimize such materials. Criticisms of the use of serum and FBS include concerns governing the ethical risks surrounding the methods used for blood co...
Photo Credit: https://pixabay.com
One of the first steps toward understanding how to patent an invention, invalidate someone else’s, or enter a new area of research is conducting a patent search. Knowing whether your invention is similar to those that already have been reported is critical before applying for a patent or beginning research. If your invention has been patented already or has been described or published anywhere in the world, then it is considered “prior art,” and your patent will be denied. Numerous patents granted in your research area can limit the scope of your research field.
Here I revise my original 2003 article on patent searching (
1
) and discuss fundamental principles regarding patents that you should know before starting your patent search. I also suggest some searching principles and list databases (both fee-based and free) that can be used to search for “prior art.”
Patent Basics
One of the basic tenets of intellectual property law is that patents are granted to advance innova...
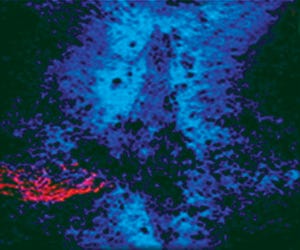
Photo 1: In this spinal cord lesion, red-dyed spinal cord nerves are trapped in a glial scar by blue-dyed CSPG proteins.
Injuries to the spinal cord can cause permanent paralysis and even lead to death, with little or no hope for patients to regain lost function after such trauma has occurred. News of my spinal cord research first came to prominence in the 1980s with a front-page story that chronicles a presentation at the annual Society for Neuroscience meeting (
1
). That reported the first time that crushed peripheral nerves had been regenerated back into a patient’s spinal cord.
Regeneration of spinal cord nerves after injury — a far more complex challenge — is coming closer to fruition as Canadian company NervGen Pharma works to develop my discoveries with an interim goal of conducting human clinical trials. At Case Western Reserve University’s medical school, my team and I are working to understand why nerves that are damaged through spinal cord injury (SCI) don’t regenerate and to identify noninvas...
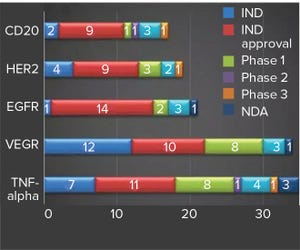
Figure 1: Hottest targets for MAb drugs under development in China (source: Advances in Biopharmaceuticals in China, 2nd Edition 2018; http://bioplanassociates.com/china)
In the past decade, the world has seen rapid growth of more than 15% in China’s biopharmaceutical market (
1
). According to the second edition of BioPlan’s Advances in Biopharmaceutical Technology in China, the most populous country in the world — with the largest patient groups — has a growing economy with its GDP second only to that of the United States. Rapid urbanization and greater access to national healthcare insurance puts China in second place after the United States for the largest market for pharmaceuticals — $122.6 billion in 2017 (
1
). With its surging middle class and increasing demand for medicines, the country is expected to sustain rapid growth throughout the next decade.
Although chemical drugs and traditional Chinese medicine both are experiencing robust expansion, it is the development of biosimilar therapeutics, es...
Figure 1: Titer in the protein A load (permeate) changes over a load interval during continuous production due to variations in cell density and cell-specific productivity in the perfusion reactor. The system shown here using two parallel protein A columns is a design currently used at BI Fremont in California, but other configurations are possible.
During production of therapeutic antibodies, harvest titer is measured to monitor product mass loaded onto the protein A capture column. This prevents both column underloading (underusing expensive resin) and overloading (wasting product as flow-through (FT)) while allowing for column yield calculations. Batch production yields a single homogenous harvest pool, thus only one titer measurement (along with volume loaded) is sufficient to determine the mass loaded. During continuous production, however, cell-free harvest (permeate) continuously exits a perfusion reactor and loads a protein A column, so the product titer can vary over the loading period (Figure 1)...
Figure 1: Vaccine process development – general approach
Scientists have made significant breakthroughs in bioprocess and analytical technologies for supporting vaccine development. Such technologies have helped vaccine manufacturers achieve consistent product purity and quality rapidly and cost effectively. Although interest in vaccine development and manufacture continues to increase because of the rapid growth of the global vaccine market, this area of the bioprocess industry remains challenging and complex.
Here we review the current constraints and complexities in the vaccine industry, specifically related to product development and manufacture. We describe the evolution of bioprocess and analytics innovations and technologies to overcome vaccine challenges, with an outlook into the future.
Challenges and Complexities
The global vaccine market has been growing rapidly and thus has been attracting interest and new players. The World Health Organization (WHO) (
1
) has reported that from 2000 to 2013, ...
Illustration of
Clostridium difficile
spore-forming bacteria, which cause pseudomembraneous colitis and are associated with nosocomial antibiotic resistance. Kateryna Kon (www.istock.adobe.com)
Scientists have discovered that microorganisms associated with the body play a critical role in human health (
1
). The intestinal microbiome is the collection of all combined genetic material of microorganisms in the intestines. An intestinal
microbiota,
a term frequently used interchangeably with
microbiome,
is the collection of such microorganisms in the intestine. With the volume of research into the human microbiome expanding, so too is the number of examples of its benefits to our health (
1
). Like diseases of the human genome and physiology, those related to the microbiome are starting to be investigated to their root causes. That has generated new interest in treating microorganisms or metabolites in the microbiome as druggable targets.
A small number of therapeutics target the microbiome, includ...
Cell line development is a critical upstream step for the manufacture of monoclonal antibodies (MAbs). Cells must be engineered to produce a biologic of interest, and the right clone must be selected to deliver material for preclinical and clinical studies. A high-producing cell line is then needed to support clinical studies and, ultimately, commercialization of the therapeutic.
Please fill out the form below to read more.
Corresponding authors are
Aurore Poles
, Global Technical and Compliance Expert, and
Guillaume Plane
, Global Development and Marketing Manager; [email protected] and [email protected]. Both focus on BioReliance® End-to-End Solutions at MilliporeSigma.
The life science business of Merck KGaA, Darmstadt, Germany, operates as MilliporeSigma in the United States and Canada.
To learn about our plug-and-play upstream development service, please visit
EMDMillipore.com/plug-play-upstream
.
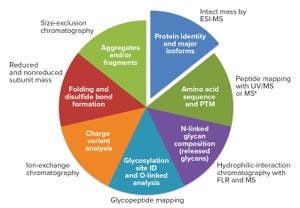
Figure 1: The analytical package; methods used for similarity and comparability assessments for biotechnology products (ESI-MS = electrospray ionization mass spectrometry; FLR = fluorescence)
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (
1
).
Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle assessments. It falls in line with the US Food and Drug Administration’s (FDA’s) totality of evidence concept (
2
) and uses the entire analytical package generated for each biotechnology product (
3
,
4
).
Analytical Package
Product charact...
When using single-use systems (SUS) to process biopharmaceuticals, preventing drug product contamination from extractables and leachables (E&Ls) and embedded particulate matter (gel particles) in the polymer films used to make bioprocess bags is critical. Using a pressure burst test to assess film integrity, Sartorius Stedim Biotech’s Klaus Wormuth and colleagues compared Flexboy and Flexsafe samples with gel-particle-free materials to assess their potential for contamination. The results showed that only large (2–4 mm2) gel particles affected the burst test results, concluding that the risk from embedded gel particles to single-use film integrity and drug purity is very low and that E&Ls were highly unlikely to reach concentrations that would require a chemical analysis.
Just fill out the form below to download this article now.
In an Ask-the-Expert webinar on 16 May 2019, Xuemei He (R&D manager of chromatography media chemistry at Bio-Rad Laboratories, Inc.) introduced the Nuvia aPrime 4A mixed-mode chromatography resin. Its macroporous particles exhibit good pressure-flow properties in addition to optimal ligand density and hydrophobicity. So this versatile resin offers effective separation of target from impurities in both flow-through and bind–elute modes, with good recovery of target proteins under gentle purification condition.
He’s Presentation
The resin is a hydrophobic anion exchanger with positively charged ligands that separate biomolecules through both electrostatic and hydrophobic interactions. The base particles are polymeric polymers with open structures that enable efficient mass transfer of desired biomolecules. The particles are strong enough mechanically to be operated at fast flow during large-scale bioprocessing.
Those properties give the resin adaptability and increased selectivity compared with traditional ...
Charles Meadows (product manager at Sartorius Stedim Biotech) joined BPI for an Ask-the-Expert webinar on 13 June 2019 featuring the MYCAP CCX cell culture expansion system. He showed how a closed aseptic cell expansion process can decrease the rate of batch failure from contamination, in turn reducing waste and overhead costs.
Meadows’s Presentation
Contamination is an inherent risk of open cell culture expansion systems. Each passage from one flask to the next potentially exposes cellular material. A recent BioPlan Associates survey noted that batch failures from contamination occur once every 9.4 months, costing US$1–2 million. Contamination is especially troubling for facilities that make <1,000-L batches, with 2.3% of them lost to culture corruption. But expensive countermeasures such as biosafety cabinets cannot eliminate the risk of contamination.
With those challenges in mind, Sartorius Stedim Biotech developed a “lean” expansion process to reduce the need for biosafety cabinets while limiting con...
Effective transfer of technology from a sponsor facility to a contract development and manufacturing organization (CDMO) is essential to rapid biomanufacturing, especially for early and late-stage clinical supply. But the process is not as simple as “plug and play.” In an Ask-the-Expert webinar on 25 June 2019, Roy Sevilla (senior manager of upstream process development at Avid Bioservices) explained how his company orchestrates transfer operations with cross-functional teams. Strong, frequent collaboration with process sponsors ultimately mitigates risks associated with scale-up, increasing the likelihood of project success and timeliness.
Sevilla’s Presentation
Successful technology transfer hinges on clear communication between sponsor and CDMO from the outset. Avid involves clients in all phases of the technology transfer process; this includes face-to-face meetings held at critical project milestones. A personalized, coordinated effort helps the CDMO gather crucial process data from the sending site ...
Campbell Bunce (chief scientific officer at Abzena) joined BPI for an Ask-the-Expert webinar on 21 May 2019 to discuss his company’s approach to developability assessment, an increasingly popular way to identify leading drug candidates. Bunce explained what developability assessment is and how Abzena uses it to identify candidate liabilities and manage both clinical and financial risk during key stages of drug development.
Bunce’s Presentation
The high attrition rate of drug candidates from discovery to clinical proof-of-concept is well known. The degree of failure makes it crucial for developers to predict risk factors that could present in later, more costly stages of manufacture and clinical evaluation. Thus, companies increasingly are turning to developability assessment, combining in silico analysis with in vivo and ex vivo experiments that characterize lead drug candidates with the highest chance of success.
Developability studies establish two primary concerns: whether a drug candidate can be manuf...
On 31 July 2019 Jamie Hall (project manager at Intertek Pharmaceutical Services) joined BPI for an “Ask the Expert” webinar to explore key applications and considerations for implementing flow cytometry (FC) methods in a GMP environment. This increasingly important technique can be applied to all stages of drug development in a range of applications, including cell-line identification, immunophenotyping, and cell-based potency assays. In a regulatory environment, such flexibility can present challenges. Hall explained how scientists can approach FC method development and validation amid rigorous analytical requirements.
Flow Cytometry Technology
FC provides comprehensive data by analyzing multiple parameters in fluorescently labeled suspensions of cells or particles. The cytometer channels the suspension into a stream of single particles that passes through a flow cell. Lasers of different colors excite the fluorochromes; mirrors and filters allow their detection. Through a combination of fluorescent labe...
Image: iStock/STILLFX
The SAMPLE program — a Standard Approach to ATMP Tissue collection — is intended to help build capacity for the UK National Health System (NHS) to expand the use of next-generation cell and gene therapies for both cancer and noncancer illnesses. Now it has been given the green light by Innovate UK (IUK), the United Kingdom’s technology strategy agency. An award through the Industrial Strategy Challenge Fund is supporting the program for standardizing how cell and gene samples are collected for modification as advanced-therapy medicinal products (ATMPs).
This project will be led by a team of researchers from The Christie, which as the largest single-site cancer center in Europe is part of the Manchester Cancer Research Centre in the United Kingdom. They will work with a nationwide consortium of NHS Trusts (including Manchester University NHS Foundation Trust, Guy’s and St. Thomas’s NHS Foundation Trusts in London, and Newcastle upon Tyne), the NHS Blood and Transplant agency, and the ...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.