April 2024 Featured Report
Antibody Engineering and Technologies Featured Report
Pharma 4.0 has initiated smart integration of digital technologies to enhance efficiency, quality, and innovation throughout a pharmaceutical production life cycle. The paradigm has transformed the industry with tools such as data analytics, the Internet of Things (IoT), enterprise software and process control systems, automation, and Artificial intelligence (AI). Rapid development of AI and machine learning (ML) applications is revolutionizing medicine in fields such as pharmacology, drug discovery, and bioinformatics
(1)
. AI brings new capabilities to biomarker and therapeutic target discovery, expediting processes and enabling developers to make well-informed decisions about their programs.
For example, AI can mine databases, digital libraries, and research publications to discover and characterize biological pathways, molecules, and individual moieties associated with disease. Its ability to provide understanding of molecular relationships advances biodiscovery and molecular development of therapeut...
Biologics play a vital role in the pharmaceutical sector and hold much promise for improving health. Approved protein drugs and biosimilars are making their largest impacts in the oncology and autoimmune domains. Monoclonal antibodies (mAbs) form the largest segment of biopharmaceuticals with complex molecular manifestations such as posttranslational modifications (PTMs). Recent approvals for bispecific antibodies (bsAbs) and antigen-binding fragments (Fabs) now highlight the importance of analytical characterization for such multifaceted entities.
Development of antibody-based therapeutics demands cutting-edge technology to characterize their critical quality attributes (CQAs)
(1)
. At early stages, intact and subunit molecular-mass analyses can provide quick and accurate glances at the CQAs of biotherapeutics undergoing development or biosimilarity assessment. Several strategies have been used to measure protein molecular mass at both intact and subunit levels, with a focus on variants. Early stage inp...
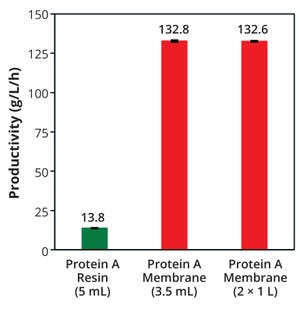
The past decade has brought a rise in the use of disposable products for biopharmaceutical downstream processing, especially in chromatographic purification
(1, 2)
. Applying such materials in bioprocessing reduces contamination risk, shortens operation times related to preparation and cleaning, and decreases operator error. As part of our investigation into the potential of single-use technologies in antibody manufacturing, we have evaluated the current generation of membrane chromatography that can operate at high flow rates to provide high productivity in a disposable system
(3)
. In testing over several months, we reviewed this modern technology to understand better the impact that it can have on antibody purification processes and what it can offer as an alternative to resin-packed columns.
We compared manufacturing performance and other data from small- and manufacturing-scale protein A affinity membrane devices, and we sought to establish equivalency with column chromatography. We found that the ...
Recombinant antibody technology addressed many problems associated with hybridoma-based platforms for biologics production, opening the door to new classes of drugs. Today, recombinant technology still contributes to the discovery and development of antibody-based therapeutics while helping to improve the performance of other biological modalities.
Early monoclonal antibody (mAb) therapies were developed using hybridoma platforms, which involve immunization of animal subjects with target antigens or immunogens. Such technologies were beset not only by ethical issues, but also by batch-to-batch variability, antibody heterogeneity, diminished antibody productivity, limited scalability, and high costs. Moreover, hybridoma-based processes took multiple months from immunization to establishment of specific clones and mAb production.
On the other hand, recombinant antibodies are generated from host cell lines using recombinant DNA technology. This approach greatly reduces reliance on animal experimentation whil...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.