GE Life Sciences January 2018 Special Report
Protein A has made monoclonal antibodies (MAbs) highly manufacturable and easier to develop in laboratories. Its use has enabled purification platform approaches, which have been key elements in growth of the MAb market. But in general, protein A columns are too large, which means they either limit the use of prepacked technology, or create a mismatch between a protein A column and downstream operations. Protein A columns also are more prone to bioburden contamination than any other step in a downstream process. The special report
Addressing High Demands for Increased Productivity in Downstream Operations
focuses on these issues and technologies and innovations that will help companies improve their manufacturing process.
Just fill out the form to view and download the complete eBook.
Figure 1:
The extreme cost of a bioburden incident
Protein A has been a fantastic tool for the antibody industry. It is without doubt the most well-established purification technique in monoclonal antibody (MAb) manufacturing today due to a few key success factors. Protein A is the result of the long evolution of Staphylococcus aureus that developed a defense system against antibodies. Protein A exists on the cell wall of about 9% of S. aureus strains and immobilizes IgGs. When there is an immune response in the body, the bacterium defends itself by disabling IgG molecules, preventing them from attacking the cells. With this natural origin, it is hard to engineer an alternative with superior selectivity.
Despite protein A’s natural origins, the bioindustry has worked to make it more useful in industrial manufacturing by improving its alkaline stability. We have even played with the structure of protein A to increase the amount of binding domains. Throughout the generations of protein A resins, this engin...
Figure 1:
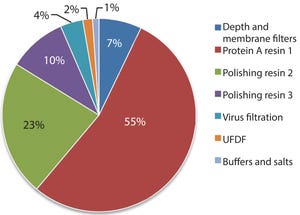
Estimated percentage of raw material costs for monoclonal antibody downstream process
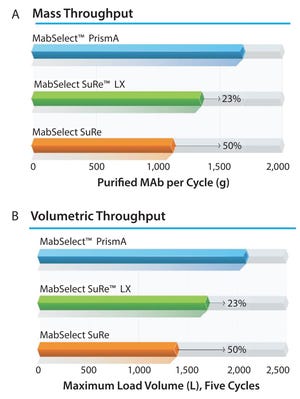
Next-generation, high-capacity, alkali-stable protein A resins have recently become available in anticipation of increased production and throughput demand for protein therapeutic processes. Efficient caustic cleaning and bioburden control regimens have allowed agarose-based, alkali-stable protein A chromatography resins to become the backbone of many commercial purification processes for over a decade and have served as the primary capture step for monoclonal antibody (MAb) purification processes. However, the relatively high raw material costs, along with limitations to binding capacity and aggressive resin stripping strategies with current resins, still represent a high contribution to overall manufacturing cost of goods. To address this, a novel higher capacity prototype protein A resin from GE Healthcare (MabSelect™ PrismA) was evaluated using a CHO-expressed MAb. Stability and performance of the resin ov...
Figure 1:
Protein A chromatography resin capacity and productivity (adapted from Bolton G, et al. The Role of More Than 40 Years of Improvement in Protein A Chromatography
in the Growth of the Therapeutic Antibody Industry.
Biotechnol. Prog.
32, 2016: 1193–1202)
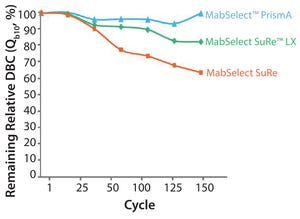
The monoclonal antibody (MAb) market has grown over the past decade to be about half of the biomanufacturing market today. This growth should continue, driven by a strong pipeline of MAbs that are currently in phase 2 and 3 clinical trials. A synergistic evolution of MAbs and protein A resins also has taken place in the market. Figure 1 shows a graph adapted from an Amgen study of the development of protein A productivity and capacity over the past 40 years. Protein A has made MAbs highly manufacturable and easier to develop in laboratories. The purification platform approach, which has been enabled by protein A, has been a key element in this market growth.
As Figure 1 shows, productivity of protein A resins has increased >4% annually since 19...
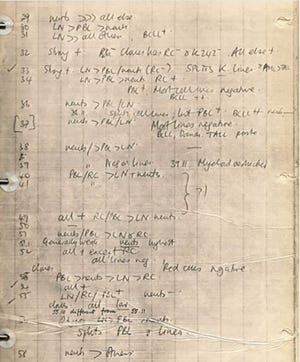
Photo 1:
A page from Herman Waldmann’s notebook
In 1980 at the University of Cambridge’s department of pathology, I worked with Herman Waldmann to develop monoclonal antibodies (MAbs) as treatments for graft-versus-host disease (GVHD). That disease is associated with serious complications of stem cell transplantation when attacking T-cells can damage the lungs, liver, skin, and other organs. If we could find a specific MAb that would work with the human complement system to kill those cells, they could be selectively removed from the bone marrow. The human complement system enables the body to destroy cells targeted with antibodies.
From a great collection of rat monoclonal antibodies of unknown specificity (Photo 1), we selected antibodies that could kill T-cells with human complements. Because we wanted to use immunoprecipitation to purify those antibodies, we further screened selected molecules for protein A binding. The selected antibody was named Campath after the Cambridge Pathology Department.
Fig...
Brian Caine (left) and Jonathan Royce (right)
PHOTO BY LEAH ROSIN
At the Biotech Week Boston conference (24–28 September 2017),
BioProcess International
publisher Brian Caine had the opportunity to speak with Jonathan Royce, global business leader for chromatography resins at GE Healthcare. Their discussion covered current downstream challenges and the company’s next-generation chromatography resin.
Caine:
What are the most critical purification challenges for biomanufacturers?
Royce:
The bioindustry has seen more than a 100-fold increase in the productivity of cells. This means that downstream purification technology must continue to evolve and meet those productivity gains so that purification doesn’t become the rate-limiting step in a process.
Biomanufacturers today seek ways to use their existing equipment more effectively and even incorporate reusable raw materials more effectively. There is a lot of focus on finding ways to get efficient cleaning of chromatography resins and to ensure that they ...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.