January-February 2018
Happy New Year! This is our first combined January–February issue, but it should give you plenty of reading material to compensate for the absence of a January issue. There are some new approaches at work for us this year that bring new opportunities for both end-user and supplier authors, as well as offering us additional options for more timely publication of high-priority information.
As you look at our table of contents, the most obvious change is the change to
sponsored
“Supplier Side” articles. Because our audited, controlled-publication model imposes strict ratios of editorial to advertising pages, we’ve been able to publish only one such paper per issue (two at most). But these articles often are timed to coincide with an event or product launch. The editorial part of the process(es) for supplier-written papers has not changed very much, but allowing for sponsorship gives us pages for more of them. This introduces a more collaborative (editorial + marketing) process that can help you get the wor...
Tracking Antibodies in the Clinic
In tracking clinical trial activity around the world, GlobalData has identified 5,273 clinical trials of monoclonal antibodies (MAbs) that started between 1 January 2007 and 31 December 2016. The top three drugs in numbers of trials were bevacizumab, adalimumab, and tocilizumab. Rounding out the top 10 were rituximab, nivolumab, pembrolizumab, ranibizumab, secukinumab, trastuzumab, and ipilimumab.
Healthcare analyst Marco Borria said, “All the top 10 drugs are marketed, and the majority of trials initiated for them were phase 3 trials, closely followed by phase 4 and phase 2.” But the largest share of bevacizumab trials were in phase 2, and 35% of all studies covered indications for which no drugs have yet been approved.
ISCT Supports FDA Regen-Med Policy
Late in fall 2017, the International Society for Cellular Therapy (ISCT) offered its support for the newly released comprehensive regenerative medicine policy framework from the Food and Drug Administration (FDA). ICT’s ...
WWW.GRAPHICSTOCK.COM
Start-ups in life sciences are constantly reshaping and redefining markets. As such, these companies must understand their unique markets because potential partners and investors seek out companies with such understanding. In my experience, it is not unusual for entrepreneurs to believe that they already know their market. They might have been active in their market for a long time, or they might have operated in similar industries and are making parallel assumptions. But markets are fluid environments, and they change rapidly. Bringing compelling arguments based on solid market research is the key to convincing people (both internally and externally) that you know your markets.
Hence, market research is conducted for two audiences. First, it helps the organization itself make optimal decisions. Market research is conducted when a lack of information will cost more than the cost to acquire that information. Second, market research is conducted to inform potential partners and investor...
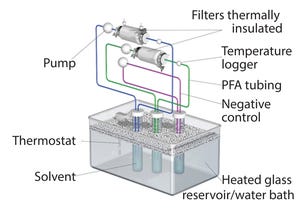
Figure 1: Filter extraction set-up (PFA = perfluoroalkoxy alkane)
Benefits of single-use technologies over traditional stainless-steel solutions in biopharmaceutical manufacturing include reductions in set-up times, cleaning/cleaning validation costs, elimination of cross-contamination risks, and smaller operating footprints. But despite increasing adoption of such systems, concerns remain about extractable and leachable (E&L) compounds from plastic single-use systems (SUS) components with the potential to compromise the efficacy and safety of final drug products. Such concerns are magnified by the growing number of SUS suppliers and the complex supply chain for SUS and components.
Although all equipment used for the manufacture of biologic drug substances and drug products must meet current good manufacturing practice (CGMP) requirements (
1
) and be free of materials that can lessen safety or efficacy of a drug product (
2
), no specific regulatory guidelines or industry standards pertain to the perform...
Figure 1A: Flow schematic of the cell expansion process; see text for details regarding process steps (numbered 1–6). Colored (upward-pointing) arrows indicate location (between steps 4 and 5) where variability in
q
P
was observed.
Variation in bioproduction is recognized in the industry and often attributed to one or more of four sources: raw materials (including consumables), operational inputs (measurements, methods, personnel, equipment), environmental factors, and biological variation inherent to living cells (
1
). Variability can occur even among replicate units regardless of production mode (e.g., fed-batch or perfusion), and it can manifest as variability in productivity, cell metabolism, and/or product quality (
2
–
4
).
In commercial biomanufacturing, meeting all product quality attributes is a requirement for regulatory compliance as well as for safe and effective patient treatment. However, a robust and well-controlled production process also must yield consistent titers.
Figure 1B: Setup o...
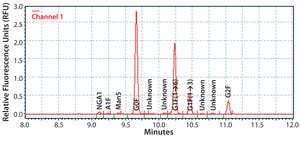
Figure 1: Electropherogram of IgG MAb 1 glycans using the HTP method
The biopharmaceutical industry needs better understanding of how monoclonal antibody (MAb) glycosylation is influenced by components in cultivation media — and it needs methods to exert some control over the structure of MAb glycans. That structure can affect MAb function. Thus, a high-throughput (HTP) assay is needed for characterizing MAb glycosylation so that developers can observe the effects of cultivation conditions on MAb glycosylation rapidly, with a goal of producing MAbs that have a desired glycan structure. The method also must be capable of testing a large number of samples in a reasonable amount of time. We established our HTP assay using 96-well plates and negatively charged magnetic beads based on a method published by Varadi et al. (
1
). Advantages of this assay include a substantial reduction in sample preparation time, a one-pot strategy for all reactions, and the replacement of toxic NaCNBH
3
with 2-methylpyridine bo...
HTTP://STOCK.ADOBE.COM
Monoclonal antibodies (MAbs) are bivalent and monospecific, with two antigen-binding arms that both recognize the same epitope. Bispecific and multispecific antibodies, collectively referred to herein as bispecific antibodies (bsAbs), can have two or more antigen-binding sites, which are capable of recognizing and binding two or more unique epitopes. Based on their structure, bsAbs can be divided into two broad subgroups: IgG-like bsAbs and non–IgG-like bsAbs. We have chosen to focus on the former in this review.
Part one
provides a pipeline update for IgG-like bsAbs; lists their general advantages and disadvantages compared with MAbs; and describes different formats, their structural characteristics, and how structural variations contribute to improved productivity (
1
). Here, part 2 goes into more detail describing how bsAbs are applied in medical research as well as basic upstream and downstream manufacturing considerations.
Comparing bsAbs and Monoclonal Antibodies
Like MAbs,...
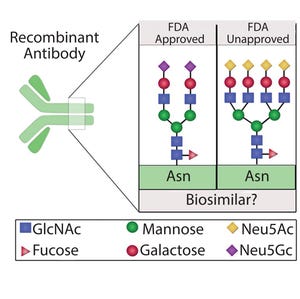
Figure 1: Different protein glycoforms; recombinant proteins expressed for use as biosimilars might not be FDA approved because of variation in protein glycosylation patterns that potentially alter protein structure, stability, and activity
The global market for biotherapeutics has expanded extensively over the past decade and is projected to account for more than a quarter of the pharmaceutical market by 2020, with sales exceeding US$290 billion (
1
). Continued expansion of the biosimilar marketplace has led to many commercial opportunities and technical challenges. The biological systems used to manufacture such drug products are inherently variable — a feature that has important consequences for the reproducibility, safety, and efficacy of the resulting products. Therefore, a prerequisite for introducing such biologics into routine clinical use is to ensure consistency of lot quality. Process understanding and consistency are critical because slight changes can lead to adverse effects such as immunoge...
The ready-to-use MaxiCaps MR system is entirely disposable and enables the integration of three, six, or nine 30-inch filter capsules.
Whether viral vectors are clarified or the bioburden after cell harvest needs to be reduced to recover antibodies, such applications in biopharmaceutical production require large filtration areas. Single-use technologies are indispensable in many such bioprocesses. Although some single-use filter assemblies have reached their limits, Sartorius Stedim Biotech has made developments to revolutionize these production steps.
Scale-Up Limitations in Single-Use technology
Conventional stainless steel process systems have been established for decades in the pharmaceutical industry. They are the basis of safe and reliable manufacturing processes for both classical pharmaceuticals and advanced biologics. Complexity and cost efficiency in biopharmaceutical production require great flexibility. Thus, most leading biopharmaceutical manufacturers are increasingly relying on modular sing...
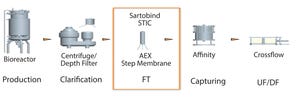
Figure 1: Placement of membrane STIC after harvest clarification step in downstream purification process (AEX = anion-exchange, FT = flow through, UF/DF = ultrafiltration/diafiltration)
Bharat Serums and Vaccines Limited (BSV) in India conducted a study based on effective removal of host cell proteins (HCPs) from a recombinant hormone with a wide isoform profile in the acidic range imparting drug-product activity. Because the hormone and HCPs have a similar range of active species, the purification process with conventional chromatography resins had difficultly removing those HCPs from the active isoforms of the hormone. To solve that issue, a membrane chromatography technique was implemented.
Our initial choice of membrane was anion exchange because we wanted to eliminate HCPs (typically negatively charged) from the bulk drug substance. One major challenge is the characteristic of higher conductivity, which usually hinders HCP clearance in flow-through mode with all the ligands for both resin- and membra...
For an industry built on constant change, there’s a surprising disconnect between the continuous drive for innovation and the inflexible facilities that house biopharmaceutical operations. Some of today’s facilities are built for today’s use with little thought about tomorrow’s.
The typical approach for a new process or drug coming to market is to start with a brand-new building and permanently embedded equipment designed around that specific process. That approach is expensive and unsustainable. New bioproduction facilities can cost US$500 million to >$1 billion and take years to bring on line, with extensive planning, permitting, construction, and qualification. Considering the rapid pace of discovery, innovation, and change in the industry, new buildings might be obsolete from the moment their doors open.
But retooling or reconfiguring facilities with their permanent equipment and structural barriers can be too expensive, time consuming, and disruptive to undertake. The result: Rather than contributing...