January-February 2023
The “happy new year” routines are pretty much over with by now, with their many variations on “shhh, don’t scare the new year away” and “2023 has got to be an improvement” among other expressions of “good riddance to 2022” and our collective anxiety over ongoing global crises. Meanwhile, as it has done since its inception, the biopharmaceutical industry continues adapting to survive and thrive as it celebrates considerable milestones. BPI devoted much of 2022 to retrospective analyses of progress during our 20 years in publication. And this new year may be, for us and many others, the one in which we can regroup, take stock of where we are, and collectively work toward accomplishing the next big things.
BPI associate editor Josh Abbott
Our November–December editorial offered farewell insights from our outgoing (now happily retired but unsurprisingly still busy) managing editor, Maribel Rios. In this issue, I am delighted to introduce you to our
new associate editor:
Josh Abbott joined our team in early ...
HTTPS://WWW.ALAMY.COM
As the bioprocessing sector marches toward the future, digitalization stands at the heart of the Industry 4.0 vision of smart, self-organizing factories. Along with critical success factors such as infrastructure investment in cloud-based technologies, digitalization enables companies to turn data into intelligence. The realization of Industry 4.0 promises digitally integrated facilities with fully automated manufacturing, real-time traceability, standardized procedures, and agile processes (
1, 2
). Here, we present the results of a benchmarking survey that drew participation from leading biopharmaceutical companies. And we describe initiatives that UCL Biochemical Engineering is undertaking to address both the immediate and long-term digital skills needs of the sector.
Benchmarking DigitalMaturity and Skills Needs
Funded by UK Research and Innovation (UKRI) and hosted at University College London (UCL), the Future Targeted Healthcare Manufacturing (FTHM) Hub has held several events...
HTTPS://STOCK.ADOBE.COM
Discovered in the mid-1960s, adenoassociated viruses (AAVs) have become the leading vector for gene therapy in recent years. In October 2012, the first European market authorization for a human gene-therapy product was granted for UniQure’s Glybera (alipogene tiparvovec), which contains an AAV1 vector for treating patients who have lipoprotein lipase deficiency. (The product has been withdrawn from the market because of limited demand.) Both gene therapies currently approved in the United States — Luxturna (voretigene neparvovec) from Spark Therapeutics, approved in 2017, and Zolgensma (onasemnogene abeparvovec) from Novartis, approved in 2019 — are AAV-based gene therapies. Most ongoing gene-therapy pipelines remain focused on treating monogenic and rare human diseases. Some patients already have realized great benefits even as these programs advance our understanding and bring hope for those with complex diseases.
An AAV’s protein shell surrounds and protects its small (25-nm), s...
Nonhuman proteins, heterologous proteins, and even small differences in protein conformation can lead to an immunogenic reaction. That is the case with antiidiotipic antibodies, which react against determinants of an antibody’s antigen-binding region (as depicted here). (
HTTPS://ISTOCKPHOTO.COM
)
Available literature abounds with case studies describing detection and identification of host cell proteins (HCPs) and other process-related impurities. In the previous installment of our review, we analyzed noteworthy studies, highlighting what they revealed about HCP immunogenicity and calling attention to topics that require further investigation. In this final installment of our four-part study, we focus on HCP risk assessment. We explore current and emerging strategies for immunogenicity prediction, then draw out key insights from the past 40 years of literature on immune responses to HCPs.
Available Analytical Methods
The quantity of HCPs within a drug substance often represents a “good strategic control”...
HTTPS://WWW.ALAMY.COM
Establishing a cell culture process across different scales and models of bioreactors involves maintaining constant scale-independent parameters such as pH, temperature, and dissolved oxygen (DO). However, nonlinear and scale-dependent criteria (impeller agitation and gas flow rates) are adjusted on the basis of multiple normalized engineering parameters to accommodate the geometrical and design differences among bioreactors (
1–3
). Normalized engineering approaches for scaling parameterization often are based on the shear generated by impeller speed and gas flow rates. Kinetic energy transmitted into bioreactor fluid — from impeller rotation and the sparging gas — dissipates in the form of heat. That heat in turn generates a velocity gradient in the fluid known as shear, which is proportional to the energy dissipation rate (EDR), according to Equation 1 below (
4
).
Equation 1
Because the EDR is predictable and can be used as a proxy to assess shear, many process scientists and eng...
HTTPS://STOCK.ADOBE.COM
Biopharmaceutical drug-substance (DS) manufacturing consists of several unit operations. Upstream production includes multiple steps in growing bacterial or mammalian cells in culture. Upstream activities are followed by a series of downstream processing units including chromatography and filtration steps for removing impurities and purifying a therapeutic molecule (
1
). All these operations are inherently complex because of the natural variability associated with growing living cells and the intricacy of purification techniques used for collecting biological products.
The US Food and Drug Administration (FDA) put forth its current good manufacturing practice (CGMP) initiative in the 1970s to minimize risks associated with pharmaceutical manufacturing and ensure product quality (
2
). That paradigm has evolved in more recent years to become the Pharmaceutical Quality for the 21st Century initiative (
3
). Both initiatives enforce pharmaceutical quality standards, ensure product co...
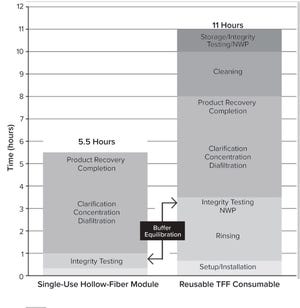
Biopharmaceutical developers and manufacturers are part of a global, dynamic, and highly competitive market. They face constant pressure to produce high-quality products within relatively short time frames and at reduced costs. Process-intensification strategies and single-use (SU) solutions are popular approaches to maximizing productivity and promoting fast, efficient, and lean processing — the pillars of next-generation facilities.
Filtration is an integral part of all bioprocesses and is applied to many up- and downstream steps, including harvest, clarification, and concentration/diafiltration. Tangential-flow filtration (TFF) is one method that represents a valuable opportunity for improvement. The increased speed and titers generated by intensified processing put additional strain on TFF operations. TFF has diverse applications, including isolating a product of interest while eliminating contaminants, exchanging buffer solutions in preparation for subsequent chromatography steps, and reducing volume...
In a November 2022 webinar, Linda Mathiasson (strategic customer lead at Cytiva) discussed the significant promise of messenger ribonucleic acid (mRNA), its role in fighting genetic and infectious diseases, and how its production can be scaled to meet the needs of different applications, ranging from large-scale vaccination to production of individual therapies.
The Presentation
Thanks to the rapid development of mRNA vaccines for COVID-19, the biopharmaceutical industry has gained momentum toward realizing the technology’s potential. The speed, flexibility, and low-cost scalability of mRNA platforms bring distinct benefits for developing vaccines against Zika virus, influenza, and diseases caused by emerging pathogens. The technology could play a role in developing cancer vaccines, cell therapies, and protein-replacement drugs. It could also be used as a delivery method for gene-editing products.
Manufacturing processes for mRNA span a broad range of scales. Large-scale operations such as those for vacci...
Contract development and manufacturing organizations (CDMOs) and biopharmaceutical companies are facing shortages in capacity for production of clinical trial material. Even as large-volume commercial biofilling capacity has increased significantly since the beginning of the pandemic, drug developers are forced to use inefficient and risky filling methods that leave them with unmet demand. John Harmer (Cytiva’s strategic initiatives leader for aseptic filling) addressed the need for flexibility and capacity for CDMOs and their clients by introducing two gloveless robotic isolator technology systems: the Cytiva Microcell vial filler and the Cytiva SA25 aseptic-filling workcell.
The Presentation
John explained that the Microcell and SA25 workcells use a robotic filling process that reduces contamination risk and provides a high degree of aseptic assurance. The system lacks glove ports, so it works without direct human intervention. Both systems have a common handling interface, requiring a small number of p...
For decades, stirred-tank reactors (STRs) have been the gold standard in cell cultivation, particularly in large-scale processes. However, rocking-motion (RM) technology offers an efficient alternative that can be a better choice for certain applications. In a November 2022 webinar, Tobias Schenk (product manager at Sartorius) discussed the two technologies, highlighting the strengths of each and how they can be used in tandem for optimal results.
The Presentation
RM bioreactor technology mixes nutrients into cell culture media using a wave motion that results in passive gassing. For instance, culture media can be infused into Flexsafe single-use (SU) bioprocess bags, which are designed to maintain cells in optimal culture conditions. Sartorius offers three options for Flexsafe RM bags: a basic bag for cultivation under constant conditions, an optical bag for processes in which pH and dissolved oxygen (DO) levels need monitoring, and a perfusion bag that is suitable for sensitive cell lines. All three for...
In a November 2022 webinar, Stuart Gibb (scientific strategy lead from Terumo Blood and Cell Technologies) explained the benefits of hollow-fiber bioreactors (HFBRs) for cell therapy. They are suitable for both adherent and suspension-adapted cells. Terumo’s bioreactors are designed to enable flexible feeding and waste-management strategies, which are key features that can facilitate optimal cell growth.
The Presentation
Gibb presented Terumo’s Quantum Flex HFBR platform, which the company launched in September 2022. Bioreactors for the Quantum Flex platform come in two sizes: the standard bioreactor with 2.1 m² of surface area and the small new bioreactor with a surface area of 0.2 m². Each is functionally closed, with material loaded and unloaded using sterile welding.
The standard HFBR contains ~11,500 semipermeable hollow fibers. Each fiber has a diameter ~200 µm, which is relatively large compared with the average chimeric antigen receptor (CAR) T cell, which ranges between
8 µm and 12 µm.
Terumo’s H...
HTTPS://STOCK.ADOBE.COM
As the world surges into the new year, pharmaceutical companies that manufacture pharmaceuticals in and/or for the United States seeking to comply with the Drug Supply Chain Security Act (DSCSA) may find themselves scrambling to meet the 27 November 2023 deadline requiring them to track and trace prescription drug products within the supply chain. Congress enacted the 60-page DSCSA in November 2013 as Title II of the Drug Quality Security Act (DQSA) to protect patient safety. That provided the pharmaceutical industry with an outline for achieving “interoperable, electronic tracing of products at the package level to identify and trace certain prescription drugs as they are distributed in the United States” (
1
). DSCSA compliance enables the US Food and Drug Administration (FDA) to protect consumers from exposure to dangerous drugs that can include counterfeits or diverted, stolen, and contaminated products. The DSCSA also facilitates how the industry manages and executes a drug re...
In late spring of 2022, the combined BPI editorial and marketing teams sent out a “Best Places to Work in Biotech” survey to BPI readers. Similar surveys appear every year from a number of other organizations. Our focus was less data driven than many others, instead inviting commentary on factors that are becoming essential to job satisfaction and employee retention.
One strong conclusion to draw from our reader responses is that people in biopharmaceutical careers want to play a key role in determining the nature and design of their working environments. Available paths to career growth and flexible working conditions are important to today’s entrants into industry positions. Respondents also want their working lives to support core social and environmental values and to provide avenues for meaningful engagement in their communities.
The prompts that we provided might reveal something about what the BPI team values about our own company, Informa, which recently was certified as a “Most Loved Workplace” (
Adenoassociated virus (AAV) vectors are made from nonenveloped virus capsids that contain single-stranded DNA. As a leading delivery system for gene therapy, AAVs are in
development to treat a number of genetic diseases (
1
). As the industry has advanced, the number of clinical trials involving such vectors has risen dramatically (
2
), increasing the need for effective manufacturing and quality control (QC) methods.
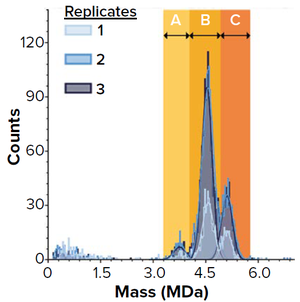
During biomanufacturing, expressed AAV capsids can incorporate both target and host-cell DNA in a heterogeneous population. Viral capsids can be empty, partially filled, correctly filled, or overfilled. The desired capsids contain a full-length transgene needed for gene therapy.
Empty, partially filled, and overfilled AAV capsids are considered to be impurities and pose risks such as competing with functional, correctly filled vectors for host-cell attachment and entry, altering drug potency, and increasing the potential for immune reactions (
3
). Characterizing the ratio of correctly fille...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.