WWW.LIFETOUCH.COM
We’ve reached midyear 2021 and our last regular issue until September. You will receive our annual Innovators issue in August. Along with it will be the (also yearly) featured report summarizing presentations from the BPI Theater at the Biotechnology Innovation Organization (BIO) International Convention.
BPI strives to maintain a balance of thought leadership from different groups in the industry ecosystem: end users, academic researchers, suppliers, contractors, regulators, and consultants. And we focus on specific bioprocesses for each product modality — with important areas of intersection and a number of notable differences. In our May issue, we stepped away from our usual phase-specific themes to examine how modern facility designs are adapting to the needs of new modalities and advancing technologies. Some of that discussion continues this month. Is it convergence or divergence (and how do we know)? Single-use technologies have moved from bench- to industrial-scale applications, d...
Massimo Dominici is scientific founder of Rigenerand srl, a joint venture between RanD (a biomedical company producing bioreactors for liver support and chemohyperthermic technology for cancer) and experts in cell and gene therapy at the University of Modena and Emilia Region in Italy. Rigenerand develops and manufactures medicinal products for cell-therapy applications (primarily for regenerative medicine and oncology) and three-dimensional (3D) bioreactors as an alternative to animal testing for preclinical investigations. The company also produces its own pipeline of cell and gene therapies for cancer treatment and operates a contract development and manufacturing (CDMO) division that provides good manufacturing practice (GMP) support for scaling up of cell-based medicinal products. Headquartered in Medolla, Italy, Rigenerand is developing a proprietary gene therapy for treating patients with pancreatic ductal adenocarcinoma (PDAC). It has been granted orphan drug designation by both the US Food and Dr...
Photo courtesy of SAFC and MilliporeSigma (EMD Millipore outside the United States)
Scott Burger and Bill Janssen are both established, independent consultants specializing in gene and cell therapies. This past spring, we three discussed several aspects of raw material strategy for advanced therapies as well as the need for trained technicians in the industry.
With a bachelor of science degree in biology from Tulane University (New Orleans, LA, 1983) and a medical doctorate from the University of Pennsylvania (Philadelphia, PA, 1988), Scott Burger served his residency training and fellowship at Washington University (St. Louis, MO) from 1989 to 1994. From there, he joined the University of Minnesota (St. Paul, MN), where he was an assistant professor of pathology and laboratory medicine from 1994–2001. There, he directed a good manufacturing practice (GMP) laboratory making cell and gene therapy products for clinical trials and transplantation. He also provided regulatory assistance to other University of...
Cell and gene therapy manufacturing is about to hit a breaking point. The tension lies between increasingly diverse research pipelines and a tradition of dedicated facilities built for single-product, large-scale manufacturing. That incompatibility is widening as more cell and gene therapy products progress toward commercial production, forcing manufacturers to make a choice: either invest in major facility modifications and complex technology transfers to keep up or break from tradition and explore the potential of multimodal manufacturing.
More than half of cell and gene therapy manufacturers plan to adopt multimodal solutions within the next two years, according to the
Horizons: Cell and Gene Therapy
industry survey
conducted by CRB last year (Figure 1). With viral vectors, autologous cell therapies, and monoclonal antibodies (MAbs) currently dominating research pipelines, those manufacturers say that flexibility, scalability, and speed to market are driving them away from dedicated facilities. The ...
Orgenesis is combining point-of-care technologies into mobile production units, as in this artist’s rendering.
Part of the advanced therapy medicinal products (ATMPs) class of therapeutics, cell and gene therapies (CGTs) can be either autologous, using the patient’s own cells, or allogeneic, using master banked donor cells. Global biotechnology company Orgenesis focuses on autologous therapies, with processes and systems developed for closed and automated processing that have been validated for regulatory-compliant production at the point of care for patient treatment. This technology could help overcome the limitations of traditionally cost-prohibitive CGT manufacturing methods that do not translate well to commercial production because of complex logistics that ultimately limit the number of patients who can afford or even access such therapies.
To achieve its goals, Orgenesis has developed a point-of-care (“POCare”) pipeline of licensed therapies that are designed to be produced and processed in closed...
Gene therapies have reemerged as promising treatments to a number of genetic illnesses. Nearly 400 gene therapy clinical trials are recruiting or ongoing in the United States for diseases such as hemophilia and spinal muscular atrophy (
1
). The primary vehicles used today to deliver such therapies to patients’ cells are viral vectors such as adenoassociated viruses (AAVs), but producing biologically active vectors for gene therapy can be problematic. One difficulty is generating vectors at the correct concentration to yield a safe and effective therapy.
In general, AVV-based gene therapies require high concentrations of viral vectors to be effective. That is because such treatments often need to be administered in low volumes in smaller areas of the body, such as in the central nervous system (
2
). Unfortunately, if the AAV concentration is too high, the AAV capsid protein itself could trigger an immune response that causes the vector to break down and render the therapy ineffective (
3, 4
). Because ma...
Advanced-therapy medicinal product (ATMP) characterization and analysis play important roles in providing chemistry, manufacturing, and controls (CMC) information for regulatory applications as well as in supporting product-release and stability studies. Each type of advanced therapy presents different analytical development challenges, so each requires specific characterization, potency, purity and identity assays. Variability in cells and among patients, multiple and complex mechanisms of action (MoAs), a general lack of readily available reference materials, and complicated analytical methods and instruments underlie the major technical difficulties (
1
).
Many techniques for analyzing cell-therapy products are not yet recognized as standardized pharmacopeial methods. Regulatory requirements are evolving around the world, and further guidance documents are expected for both the United States and Europe in the near future. Thus, product validity should be demonstrated for regulatory authorities through ...
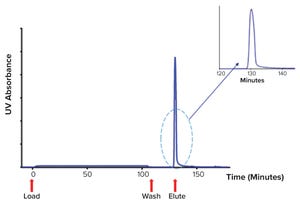
Figure 1: Chromatogram showing platelet releasate impurities being washed from a ligand-based exosome affinity purification (LEAP) column, before a buffer change eluted the purified EVs from the column
Novel therapeutics based on extracellular vesicles (EVs) recently passed a critical development milestone. During 2020, some of the first experimental EV products developed by biopharmaceutical companies entered human clinical trials (
1–3
). EVs are nanometer-sized, lipid-wrapped spheres released by almost every cell type in the human body. EVs are loaded with a cargo of proteins, lipids, and RNA, and they are tagged with surface markers that favor uptake by target cells. Thus, EVs are a key mode of cell-to-cell communication (
4
). As such, they offer intriguing potential to be used therapeutically.
Naive EVs (NEVs) isolated from sources such as platelets and stem cells are of great interest in regenerative medicine. Possible applications of NEVs include wound healing and osteoarthritis treatment (
5
). T...
The term
artificial intelligence
(AI) has become pervasive in conversations about the future of healthcare. AI has the potential to transform medicine through novel models of scientific discovery and healthcare delivery, ultimately leading to improved individual and public health. Yet misunderstanding and miscommunication abound. Thus, concepts related to AI need to be defined and explained to elevate our general level of understanding and our discourse around the topic.
The Promise of AI in Healthcare
AI has been studied by computer scientists for over 70 years. The term itself was coined by John McCarthy in 1956 at the first workshop on the subject at Dartmouth College (
1
). But the theory and topics that became known as AI have a much longer history (
2
). Even so, it remains one of the most complex and misunderstood topics in computer science because of the vast number of techniques used and the often-nebulous goals being pursued.
AI and healthcare have been bound together for over half a century. ...
Corning HYPERStack 36-layer and 12-layer cell culture vessels
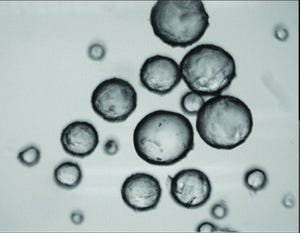
Mesenchymal stem cells (MSCs) are used frequently for cell therapy applications. As multipotent cells, they can differentiate into other lineages such as adipocytes, osteocytes, and chondrocytes. Additionally, they are known to secrete trophic factors that can play important roles in immunoregulation. Although MSCs can be isolated from several different tissue sources, those derived from bone marrow commonly are studied because they are easy to access in quantities large enough for therapeutic dosing (2 × 10
6
cells/kg of body weight). Still, that equates to 140 million cells for a 150-pound individual. And the process of expanding MSCs to achieve such quantities can introduce risks for heterogeneity-induced quality failures. Chances of clinical success can improve with a manufacturing process that maintains a homogeneous MSC population after expansion to meet required critical quality attributes (CQAs).
Once cells are scaled up, they need to ...
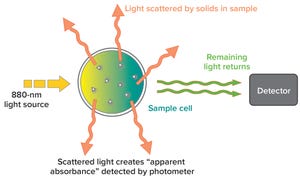
Figure 1: Example of a forward light-scatting turbidity measurement system
Turbidity
describes the relative clarity of a liquid as the result of suspended solids. Instruments that measure turbidity typically use a beam of light to detect particles by measuring the difference between the amount of light emitted from the light source and the amount that is received by a detector. Such measurements are affected by the size, shape, and number of particles in a sample of liquid because those solids scatter the incoming light, which provides an apparent absorbance that is measured by the detector. By contrast, PendoTech’s turbidity measurement system uses “forward light scattering” to measure turbidity: It detects the scattering of light that passes straight through a sample (Figure 1).
In bioprocess operations, the turbidity of a fluid often is measured to monitor unclarified material that leaves a bioreactor or fermentation vessel. Turbidity measurements also are taken to analyze filter performance. Those me...
Rapidly growing interest in gene therapy has led to the need for more cost-effective and scalable viral-vector manufacturing platforms. Adenoassociated virus (AAV) has become a vector of choice because of its safety profile (nonpathogenic infection). In addition, AAV cannot replicate on its own and is not integrated directly into the host genome.
AAV vector manufacturing using human embryonic kidney (HEK) cells in either adherent or suspension mode includes several typical processing steps: cell expansion, plasmid transfection, viral-vector production, cell lysis, purification, and fill and finish. The purification process usually involves clarification, capture through affinity chromatography, polishing with anion-exchange (AEX) chromatography, tangential-flow filtration (TFF) concentration/diafiltration, and final filtration. An additional TFF process step often is added before the affinity step to concentrate the product stream.
Those upstream and downstream processes can be fairly straightforward at s...
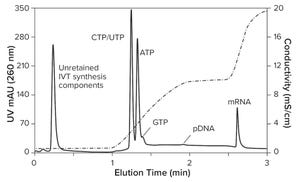
Figure 1: IVT analysis after four hours of incubation (CIMac PrimaS, see “IVT Characterization” box for conditions)
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (
1
). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and maximize productivity.
A Complex Process
This complex process begins with a DNA plasmid (pDNA) produced in
Escherichia coli
and its subsequent purification. The process continues with in vitro transcription (IVT), followed by purification of the mRNA.
With the target DNA sequence defined a...
Mitochondria originated millions of years ago, likely when a eukaryotic cell incorporated a bacterium. Thus, mitochondrial DNA strands are 200,000× smaller than those from a cell’s nucleus. Depending on cell type, thousands of mitochondria can reside within the walls of one human cell. The scientific community long has considered these organelles to be cellular “energy factories.” But their centrality to human health and disease only now has come to light.
In a breakthrough study published in Cell (
1
), biologists collaborated with scientists from the US National Aeronautics and Space Administration (NASA) to analyze the largest collection to date of biomedical data from astronauts. As lead author Afshin Beheshti (bioinformatician and principal investigator at KBR) later reflected, da Silveira et al. sought to understand negative effects of spaceflight on human physiology (
2
). They stumbled upon “a universal mechanism that explains the kinds of changes [that occur] to the body in space” but found it “i...
Cold chain distribution is complicated and critical for formulations that must be kept in very cold temperatures in the pharmaceutical industry, since their stability decreases quickly at room temperatures. The World Health Organization (WHO) has reported over 50% of vaccines are wasted and must be disposed of globally every year due in part to disruption of the cold chain distribution and lack the resources to support the ultracold temperature requirements. A possible solution to the existing problem is Hyalo Technologies’ Hyalo Matrix sterilization method. Cold chain logistics could be eliminated by allowing for room temperature product stability, storage and transport. This method also has the ability to sterilize pharmaceutical products and medical devices at lower temperatures, which prevents product denaturation, maintains efficacy, and increases shelf life of the products. After the Hyalo matrix is added to a product it can be sterilized at the end of a manufacturing process, which will do away wit...
Although buffers are among the simplest materials used in bioprocessing, they are critical to biopharmaceutical manufacturing success. Buffer preparation, storage, and handling can require significant investments in time, labor, equipment, and facility space.
Jenny Dunker
, MSc, and
Alexander Troken
, PhD (global product managers for customized bioprocess solutions and for process liquids and buffers, respectively, at Cytiva), delivered an “Ask the Expert” presentation on 30 March 2021 to explore strategies for intensifying buffer management.
Available Options
Biopharmaceutical manufacturers often prepare buffers manually. However, 2020 market analyses from Cytiva and BioPlan Associates show that drug companies now cite buffer management as a significant constraint on production capacity. A primary challenge is that buffer tanks occupy considerable facility space — and for much longer than other kinds of equipment. In-house preparation is time- and labor-intensive, with high potential for variation acro...
Host-cell proteins (HCPs) must be assessed early in biopharmaceutical product development to establish the efficiency of a purification process. Liquid chromatography coupled with mass spectrometry (LC-MS) is a strong orthogonal method for HCP identification and quantitation. During an 18 March 2021 “Ask the Expert” presentation,
Christina Morris
(senior scientist at BioPharmaSpec) explained how MS analyses can enhance process development (PD), then presented considerations for generating spectral libraries, selecting samples for quantitation, and reviewing data.
Morris’s Presentation
Spectral Libraries:
A typical MS workflow comprises spectral-library creation, sample analysis, and data review. Libraries are constructed using information-/data-dependent acquisition (IDA/DDA), which collects HCP data with high confidence because fragment ions observed during a run can be attributed to defined precursor signals.
Typically, IDA/DDA analysis is performed on
host-cell lysate
. Doing so generates a library...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.