This year’s WCBP Symposium, an annual event presented by our friends at the CASSS organization, was my first business trip as editor in chief. Each January, WCBP brings industry, academic, and regulatory representatives together at the historic Mayflower Hotel in in Washington, DC. The theme for 2024 was “Embracing Revolution,” and it struck a chord with me in this time of great personal change after many years of relative consistency.
WCBP speakers and discussion leaders focused on chemistry, manufacturing, and controls (CMC) development approaches; innovative analytical methods; next-generation therapeutics and vaccines; and global regulatory strategies. We all enjoyed a wonderful reception at the International Spy Museum, where I magically kept running into just the right people I needed to speak with at every turn. And that week I also got to meet our new associate technical editor in person for the first time. Talk about a revolution: I never would have thought we’d hire someone without having done s...
Health authorities are turning to digitalization to streamline their processes in response to global crises (such as the COVID-19 pandemic) that have affected the supply chain. The growing complexity of information and data in regulatory submissions has encouraged health authorities to implement structured data submissions and centralized e-submission processes through shared workspaces (
1, 2
). Some ongoing initiatives seek to simplify review and assessment processes through increased global cooperation and harmonization.
As of 2024, the European Medicines Agency (EMA) is exploring revised transparency rules in its Clinical Trials Information System (CTIS) to simplify and expedite clinical trial application (CTA) approval processes (
3
). Meanwhile, the US Food and Drug Administration (FDA) is moving toward structured-data systems with its pharmaceutical quality/chemistry, manufacturing, and controls (PQ/CMC) initiative and Knowledge-Aided Assessment and Structured Application (KASA) computer-assisted r...
The International Biomanufacturing Network (IBioNe) is a group of organizations that is dedicated to spreading knowledge about biomanufacturing. IBioNe is sponsored by the US National Science Foundation (NSF Award# 2114716: principal investigator Michael Betenbaugh and co-principal investigator Seongkyu Yoon) and serves other NSF-sponsored organizations, including the Advanced Mammalian Biomanufacturing Innovation Center (AMBIC) and the Membrane Science Engineering & Technology Center. According to the IBioNe website, the network seeks to serve as “a catalyst for technology innovation in biomanufacturing with increased biomanufacturing training and workforce development opportunities globally, accelerating discoveries and developments of lifesaving drugs and vaccines.” By sharing information and training industry professionals around the world, IBioNe seeks to help make life-saving treatments available and affordable.
In November 2023, Seongkyu Yoon (co-PI of IBioNe, and chair of workforce development com...
The aims of our research have been to define and identify the specific expertise and skills gaps relevant to the biopharma 4.0 transition and to help the industry implement strategies to mitigate operational disruptions in the manufacturing of critical drug products. To do so, we used the Lightcast labor analytics database to identify real-time labor-market trends and the occurrence rate for specific skill and expertise terminology, executing a customized search by applying different filters.
Part 1 of this report described the biopharmaceutical industry’s transformative shift with the adoption of industry 4.0 principles. We identified existing key skills in the sector and the emergence of alternative skills that will be required to harness automation and digitalization. And we characterized the hypothesized skills gap as a critical challenge for which the complexities of biopharma 4.0 must be navigated to provide solutions. A “Methodology” section illustrated how we determined the skills gaps, primarily ...
Clarification of bulk cell-culture broth is the first step in biologics purification. Solid particles such as cells and cellular debris are removed, and the resulting material is processed through 0.2-µm filters in preparation for primary affinity-chromatography steps. Typically, clarification involves either two-stage depth filtration or centrifugation followed by one-stage depth filtration. Beckett reports that although two-stage, cellulose-based depth filters are an established clarification technology, associated cost and space constraints limit depth-filtration–only processes to culture volumes of 4,000 L (
1
). For larger capacities, biomanufacturers prefer to use a disc-stack centrifuge (DSC) for the first clarification step (
1
).
DSC processes can be scaled up by maintaining a constant ratio of centrifuge feed-flow rate (
Q
) to gravity equivalent setting area (
Σ
). The latter variable represents the area required for particle settling under gravity for specific operation conditions and equipmen...
Antibodies play a crucial role in the human immune system, giving their therapeutic use and utility enormous potential in many respects (
1
). Thus, the production of antibodies and their derivatives — e.g., fusion proteins based on the fragment crystallizable (Fc) region — has been the focus of the biopharmaceutical industry for decades. Development of purification technologies for monoclonal antibodies (mAbs) made them feasible as a therapeutic product class (
2, 3
). As product concentrations in the one- to two-digit g/L scale have become state of the art, the upstream mAb production process has become less limited over time (
4
). By contrast, downstream processing must be optimized to handle such high expression titers and to reduce associated costs (
5–7
).
Today’s standard mAb purification processes consist of at least two chromatographic steps, with protein A affinity chromatography often used as an initial capture step because of its high affinity and selectivity for the Fc region of antibodies (
Biomanufacturing equipment, controls, and testing procedures improve over time in ways that help to reduce the possibility of introducing adventitious viruses into biologic production. Viral contamination of biological products is a real concern. In the 1980s and 1990s, hepatitis and human immunodeficiency virus (HIV) were transmitted to patients through contaminated plasma products (
1, 2
). Viral removal and inactivation steps had not been implemented for plasma-fractionation, and donor-screening procedures for blood-borne viruses were absent (
3
). That failure resulted in many deaths and long-term illnesses. One organization that has focused on viral contamination is the Consortium on Adventitious Agent Contamination in Biomanufacturing (CAACB). It has collected comprehensive data on viral contaminations of cell-culture operations from member companies and institutions. The organization has identified 26 contaminations over
36 years of biologics production in mammalian cells (
4, 5
). To my knowledge,...
Cell culture media play a crucial role in upstream process (USP) development. Their compositions are made of complex mixtures that affect the entire development process as well as product quality. Yaron Silberberg (chief scientist at Ajinomoto CELLiST Korea) discussed a multiomics (digital twin) technology that can identify crucial bioreactions and media components by creating global network visualizations of metabolic pathways. The genome-scale metabolic model (GEM) can simulate >7,000 individual bioreactions to better represent cells, identify bottlenecks in development, and improve bioprocess productivity.
Silberberg’s Presentation
The traditional method of culture media development is to perform multiple wet experiments and statistical analyses. However, that technique doesn’t provide an in-depth view of cells, and it would take thousands of experiments to examine all correlations between media components and cell culture performance. The use of multiomics replaces experiments with computer-based simu...
Lentiviral vectors (LVVs) are crucial for long-term and stable gene expression to both dividing and nondividing cells. However, LVVs are difficult to process because of their high sensitivity to certain factors such as low pH, high salt, temperature changes, and shear forces. Sujeong Yang (senior research and development scientist at Astrea Bioseparations) introduced the LentiHERO platform, which helps users address challenging LVV purification steps, during a recent BPI webinar. The platform incorporates composite electrospun AstreAdept nanofibers, which use an expansive flow path and mild conditions to circumvent purification difficulties and achieve high yields of functional LVVs.
Yang’s Presentation
Scalable strategies are necessary to remove the high levels of contaminants, such as host cell proteins (HCPs) and double-stranded DNA (dsDNA), that are found in suspension cultures. Doing so requires the development of clarification steps prior to purification. Yang’s team conducted laboratory-scale studi...
The design and build of a facility to support clinical and commercial manufacturing of advanced therapies is fundamentally important to the success of those projects. In many cases, retrofitting an existing biomanufacturing facility (e.g., for traditional biologics manufacturing operations) can introduce significant risk to manufacturing processes for advanced therapies. Chris Berger (executive director, quality viral vector at Avid Bioservices) discussed the advantages of Avid’s Build It Right design philosophy for viral-vector contract development and manufacturing organization (CDMO) facilities.
Berger’s Presentation
Berger emphasized the importance of envisioning an entire project, start to finish, early in the life cycle to increase its chance of success and minimize rework. Project leaders should consider the scope of the work for the projects in the facility. That can include the type and scale of cell lines they are using and whether the facility will make drug products in addition to drug substan...
Most upstream processes use either batch or fed-batch methods because they are established techniques that have been validated over time. “People are hesitant to change because they feel the new intensified processes to be more complex and difficult,” said Muhammad Shamim (field application scientist from Repligen) in a December 2023 BPI webinar. However, batch and fed-batch methods have the disadvantage of fixed yields in finite culture durations. Shamim presented alternating tangential flow (ATF) and tangential-flow depth filtration (TFDF) systems for upstream process intensification. Such systems are designed to generate comparatively higher yields from longer processes.
Shamim’s Presentation
Although improved batch-mode technologies can bring some productivity gains, a fully intensified cell-culture process is needed for maximum output, and continuous capture is necessary to optimize product yield. Shamim introduced the XCell ATF and KrosFlo TFDF systems from Repligen. Both systems can be used to inte...
Demand continues to grow for supply of extracellular vesicles (EVs) such as exosomes that play an important role as intracellular messengers. However, EV manufacturing comes with challenges because of the material’s complexity and fragility. Elie Zakhem (senior manager process development at RoosterBio), Lauren Torres (field application scientist from Repligen), and Jeremy Neidert (bioprocessing account manager from Repligen) presented an Ask the Expert webinar about scaling up EV manufacturing, which necessitates using well-designed upstream and downstream platforms to ensure reproducible yields.
The Presentation
RoosterBio partnered with Repligen to develop robust, scalable manufacturing processes for EV-based therapies that would be affordable and accessible to patients. The companies used RoosterBio’s industrialized EV production platform and Repligen’s scalable and low-shear filtration technologies. EVs were produced using RoosterBio’s high-quality mesenchymal stem cells (MSCs), proprietary growth me...
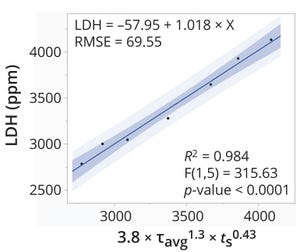
Sterile filtration is a pivotal process step in pharmaceutical manufacturing to ensure the sterility of injectable drug products. It eliminates microorganisms and particulates while safeguarding the integrity of a final product. Effectively managing and monitoring the sterile filtration process requires meticulous attention to manufacturing controls and encompasses key parameters such as flow rate, temperature, use time, and pressure. Pressure emerges as a critical factor, necessitating oversight to validate the efficacy of a filtration system and uphold the stringent standards of regulatory bodies such as the US Food and Drug Administration (FDA) and the European Commission.
Pressure monitoring of sterile filtration steps is a regulatory expectation for process design and is established during filter validation. According to the FDA’s 2004 guidance on aseptic processing, pressure, flow rate, and other factors that can affect filter performance and validation should be conducted using worst-case condition...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.






.png?width=300&auto=webp&quality=80&disable=upscale)





