Along with products and processing operations moving upstream and downstream, they also move toward the mainstream. The ramifications can become so interconnected that it is hard to discern cause and effect. One example from the past 10 years is single-use materials moving into larger scale processing. That in turn has driven much exploration into flexible operations and variations of continuous processing — neither particularly new concepts, but both now taken into more (potentially) economical directions. Related discussions are bringing heightened attention to sustainability, costs/pricing, and the future of personalized therapies and vaccines. Single-use options and automation are now essential to achieving the cost savings needed to drive down the price of final products.
Single-use technologies drive attention to ongoing factors behind industry successes. We’ve long talked about ensuring the quality and consistency of incoming raw materials and ensuring their sourcing throughout a product’s life cyc...
Niche Disease: Epidermolysis Bullosa
by Cheryl Scott
Epidermolysis bullosa (EB) is a group of rare diseases that cause skin to blister easily. In severe cases, blisters may even be internal (e.g., in mucosal tissues or organs). More than 300 mutations have been identified in this condition. Most of the many types of EB are inherited, and the condition usually manifests in infants and young children; some patients don’t develop symptoms until adolescence or early adulthood. Exceptionally mild cases may remain undetected.
Human skin has an outer layer (epidermis), a middle layer (dermis), and a lower layer (subcutis). Protein anchors prevent shearing between the top two layers. In EB patients, certain proteins are missing, allowing skin layers to separate with even minor mechanical friction. Patients have compared the blister/sore pain to that of third-degree burns. Increased risk of skin cancer is one potential complication.
EB simplex
is the most common form, typically affecting hands and feet, involving...
Table 1: Risk-level matrix
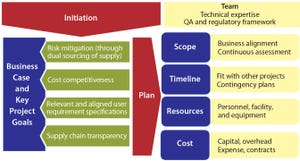
Ensuring a continuous supply of safe medicines is a key objective for the pharmaceutical industry and health authorities alike. A critical component to that end is maintaining a reliable supply of qualified raw materials (RMs) used in drug production. However, changes in suppliers, their processes, their providers, and consequently the materials they supply can occur (for a number of reasons) at any time during the life cycle of drug production. A product-supply organization therefore must be prepared to address such changes in time to prevent interruption of patients’ supply.
The biomanufacturing industry recognizes that it needs to apply a risk-based RM control strategy in line with pertinent regulatory and other guidelines based on an understanding of possible effects on product quality (
1
). Here we present a risk-based, bestpractice approach and case study for mitigating the risk of drug-product supply shortages based on the availability of qualified raw materials.
Our App...
BIORELIANCE (WWW.BIORELIANCE.COM/US)
Stability testing is required for all biopharmaceutical drug products to detect all changes in identity, purity, and potency as a result of a number of environmental and processing factors. Whether testing is conducted in-house or through contact laboratories, it involves the development and performance of comprehensive and specific stability protocols for all stages of a product’s life cycle (
1
). Testing product stability in-house requires signficant time and resources, and carries challenges associated with commercialization market, time, and capacity.
Market:
The type of stability tests that are conducted depend not only on a product and its intended use and stage of development, but also where that product will be used and stored. Global regions have been divided into five climatic zones (Table 1). Manufacturers must factor in the costs of additional stability tests when deciding on local- and/or global-market product distribution.
Table 1: Climate zones for dru...
Photo 1: A single-use quaternary pump chamber (Quattroflow) coupled with genderless connectors (AseptiQuik, CPC) for sterilemanufacturing systems
The rapid spread of contagious and lethal diseases worldwide has driven bioprocess suppliers to develop technologies for use in producing disease treatments and vaccines. Bioprocessors need to develop new biologics as well as rapid and reliable methods for bringing those treatments to commercialization. Implementing modular process solutions and single‑use handling systems in closed‑manufacturing processing is one approach to addressing those needs.
Developing and discovering solutions for meeting global healthcare conditions is an evolving part of bioindustry. As points of reference, vermin control and improved sanitation were keys to fighting plagues of centuries past. Penicillin successfully treated pneumonia during the world‑war eras. And virus‑based vaccines that treated diseases such as polio opened the door to prevention through enhanced immunity. Drugs a...
The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of the forum meetings are published in
BioProcess International
and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment will continue to be safe and efficacious.
This special report series highlights five general subject areas that have been covered in the first 10 years of the CMC Strategy Forum series: quality by design (QbD) and risk management; manufacturing strategies; analysis and characterization; assays, biosimilars, and comparability; and process- and product-related impurities. Appearing quarterly throughout 2015 and 2016, these topics will be r...
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, Biosimilar Products: Scientific Principles, Challenges, and OpportunitiesSpecial Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, Biosimilar Products: Scientific Principles, Challenges, and Opportunities
The Chemistry, Manufacturing, and Controls (CMC) Strategy Forum held on 22 January 2012 in San Francisco, CA, focused on selected scientific and regulatory aspects in the development of biosimilar products. Such products are an increasingly important area of interest for both the biopharmaceutical industry and its regulatory agencies. Biosimilars are highly complex, so scientists have been unable to demonstrate identity to a level typically possible for small molecules. Consequently, specific scientific and regulatory approaches are required to ensure the high degree of similarity sufficient to reflect the safety and efficacy of reference products.
The purposes of this forum were to highlight scientific and regulatory challenges for developing and assessing biosimilar products and to discuss industry opportunities. Presentations included case studies of experiences gained with the first biosimilar products (e.g., in Europe and Canada), examples addressing recent efforts in developing biosimilar monoclonal...
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, The Role of Higher-Order Structure in Defining Biopharmaceutical QualitySpecial Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, The Role of Higher-Order Structure in Defining Biopharmaceutical Quality
Cosponsored by CASSS (an International Separation Science Society) and the US FDA, the 17th CMC Strategy Forum was designed to explore the relationships between higher-order molecular structure and quality of therapeutic proteins and peptides, vaccines, and blood-derived products. Understanding those relationships is important to defining and controlling the critical quality attributes (CQAs) of biopharmaceutical products. The forum program highlighted the current state of the art for analytical tools used to monitor higher-order structure. Case studies demonstrating the effects of changes to higher-order structure on biological function illustrated approaches to defining correlations. Presentations by experts from regulatory agencies, academia, and industry were followed by discussions focused on correlating data derived from analytical tools to biological functions of molecules.
Regulatory Perspectives
Proteins are complex, three-dimensional (3D) structures capable of considerably changing their conform...
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, Demonstrating Comparability for Well-Characterized Biotechnology Products: Early Phase, Late Phase, and PostapprovalSpecial Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, Demonstrating Comparability for Well-Characterized Biotechnology Products: Early Phase, Late Phase, and Postapproval
Challenges and approaches in demonstrating comparability of a well-characterized biotechnology product after manufacturing changes can be as varied and complex as the products themselves. Participants at the January 2005 CMC Strategy Forum sought to discuss and agree on common implementation strategies for different manufacturing change scenarios (
1
). Development of flexible, comprehensive approaches in strategy development addressed evaluation of critical product characteristics, appropriate process steps to test, numbers of lots and levels of testing required, and assessment of product comparability. The change scenarios discussed can occur throughout the life cycle of a product from early development through postapproval manufacturing.
Early stage development is where the foundation for assessing comparability begins, and the effects of good or poor development will carry throughout a product life-cycle. Sufficient process and product knowledge is required for reliably predicting and assessing the im...
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, The Roles of Bioactivity Assays in Lot Release and Stability TestingSpecial Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, The Roles of Bioactivity Assays in Lot Release and Stability Testing
A January 2007 CMC Strategy Forum on the roles of bioactivity assays in lot release and stability testing was held in Washington, DC (
1
). Its purpose was to promote an understanding of the design and utility of bioassays throughout product development and to delineate the conditions under which surrogate assays could be used to determine product potency. Topics of discussion included appropriate assay selection at each stage of product development, the potential use of binding assays for potency testing, and the conditions under which multiple surrogate assays might be needed. A second forum in Paris (April 2008) followed up and expanded discussion on these same topics.
Case studies were presented on the role of potency assays in correlating product biological activity to structure and mechanism of action (MoA), development and use of surrogate assays for monoclonal antibody (MAb) testing, and replacement of bioassays with binding or physicochemical assays for complex biological products. Perspectives w...
WWW.PHOTOS.COM
Precious patient samples, contamination concerns, and limited product purification options have compelled manufacturers of cellular immunotherapies (iTx) such as chimeric antigen receptor T cells (CAR-T) and T-cell receptor (TCR) technologies toward the disposables industry. Such companies are implementing single-use technologies (SUTs) almost exclusively (
1
). But despite the dominance of disposable bioprocess platforms and their extraordinary growth in the iTx marketplace, researchers have made limited efforts to understand the perennial and critical bioprocessing risks of leachables and extractables.
Here we outline the potential impact on iTx of leachables and extractables and discuss relevant regulations, guidelines, and risk-assessment approaches. Fortunately, biopharmaceutical producers of monoclonal antibodies (MAbs) and recombinant proteins have been investigating the consequences of leachables and extractables on manufacturing processes (
1
–
3
) for years. iTx companies (and the...
Photo 1: SmartGlass bioreactor with the G3Lab Universal controller
Animal cell lines (the dominant expression systems in biopharmaceutical production processes) are mostly cultivated in stirred bioreactors (
1
). Although such bioreactors are widely accepted and applicable over a wide range of scales, engineering data for these systems are still lacking. Nevertheless, studies have shown that the correct choice of key parameters (e.g., power input, tip speed, mixing time, and oxygen mass transfer) can influence the growth of animal cell cultures (
2
). Therefore, detailed characterization is essential. It enables reliable scaling up of production processes and can be used to compare different bioreactors.
Finesse Solutions, Inc. has commercialized its laboratory-scale SmartGlass bioreactor (Photo 1). The system has a maximum working volume of 2 L and is agitated by combined two-stage axial-radial flow impellers. A Finesse G3Lab Universal controller, powered by Finesse TruBio software, operates the bioreact...
Sponsored Content
with Serena Fries Smith (Thermo Fisher Scientific)
In the early 2000s, maximizing expression titers was the industry’s biggest challenge. Over the past 10–15 years, significant advances in media and feeds have enabled standard fed-batch processes to achieve 3 g/L, some as high as 7 g/L. The productivity increase is enabling scientists to move beyond titer and focus resources on addressing other scientific challenges (e.g., product quality modulation and process scale-up).
One way to address those challenges is by leveraging feed solutions to target specific protein quality attributes and simplify manufacturing. In a 1 October 2015 webinar, Serena Fries Smith explained how changes in feeding regimen and composition can enable companies to “dial in” specific glycosylation patterns. Her company has created superconcentrated feeds to maximize nutrient addition while minimizing bioreactors volume changes. Below is a summary of her presentation. You can find more detail and the full slide presentation online.
S...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.