One of our annual tasks as a publication staff is to develop next year’s editorial calendar. How well are we anticipating new directions in research, development, and manufacturing? Are we including interesting and forward-thinking topics — or relying too heavily on frequent, overintroduced themes? We have seen an overall generational change in conference attendance and our reading audience. So we also ask ourselves whether we are correctly assessing information needs and providing accessible levels of detail.
It’s not a perfect system and often feels like fortune telling. Big themes are about the same as ever and generally concern process and product development. Special themes have for a few years now included single-use technologies, regenerative medicine, and methods of intensifying and integrating processes to reduce operating footprints and time to market. So we look for nuances, specific applications that can serve as case studies, and new voices (new authors and fresh insights).
Because our editor...
Figure 1: Supplier side capabilities over time; this chart represents major companies’ strategic intents; positions shown are relative, not scaled by market values, and vary by geography and technological capability.
Only a thin line now separates biopharmaceutical manufacturers and suppliers because the latter are increasingly becoming the process knowledge owners in the biopharmaceutical industry. As a result, suppliers are racing to become the most efficient “total solutions” provider.
In the 1990s, leading players in the industry such as Pall, Millipore, and Sartorius all supplied membrane filters for upstream and, to some extent, downstream processes with their crossflow and final filtration offerings. Pharmacia (which became GE Healthcare) was the major force in the final purification chromatography segment. A host of suppliers in the laboratory equipment segment occupied the discovery space, including Fisher Scientific (now Thermo Fisher Scientific) and VWR (now a subsidiary of Avantor).
Another se...
Figure 1: Major hurdles facing the bioprinting field; findings based on primary and secondary research by Charles River Associates (CRA).
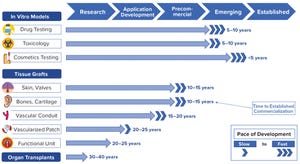
Bioprinting has advanced rapidly through engineering step changes in the use of three-dimensional (3D) printing. With these developments, living cells can be positioned layer by layer to produce functional tissue structures. Key attributes of this emerging technology are its high scalability and modularity, which enable automated and repeatable manufacture of a wide variety of tissues. These high-throughput biofabrication capabilities equip companies with tools to develop 3D-printed tissues for broad applications, from in vitro drug testing models to therapeutic tissue implants, to whole organ transplants.
Promising Developments
According to Jay Hoying, chief scientist at Advanced Solutions Life Sciences, “The regenerative medicine field is seeing an explosion of novel tissue and tissue-model fabrication strategies, with bioprinting as a mainstay approach. Translating t...
Scientist writing test results while standing in the lab. Protective suit and gloves on.
Over the past 24 months, the leadership of the Bio-Process Systems Alliance (BPSA) has initiated a pilot program to demonstrate the joint feasibility of collecting and sharing pertinent industry business data. We began with “quality-complaint data” centered on the premise that product complaints are tightly associated with product-quality defects in single-use systems (SUSs). This approach can be viewed as “risk assessment 101.”
BPSA’s Representation
of the Single-Use Industry
BPSA is an industry organization primarily comprising drug manufacturers, single-use system suppliers, SUS component suppliers, specialty test laboratories, and educational institutions. The organization’s goals are to “advance the adoption of single-use worldwide” through technical guides, national and international conferences, and advocacy efforts around and within industry standards groups and regulatory bodies; and through providing a saf...
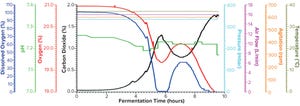
Figure 1a: Fermentation profile of protein A in the Sartorius SIP system at 10-L scale (dO
2
in blue, pH in green, O
2
in red, CO
2
in black, pressure in cyan, air flow in magenta, agitation in orange, and temperature in olive); all controlling parameters in
y
-axes on the right were kept constant throughout growth. The
y
-axes on the left showed parameters mainly for monitoring, which changed through the fermentation process.
Single-use bioreactors have become widely accepted and well established for cell culture applications in the biopharmaceutical industry for over a decade (
1
). Abbott Diagnostics has moved into this technology already for commercial production of some biologic molecules. However, single-use systems (SUSs) are rarely available for microbial applications, mostly because of the technical challenge in designing cost-effective SUSs that can meet high oxygen transfer needs and remove excessive heat generated during fermentation. Thus, an important part of our biologics manufacturing...
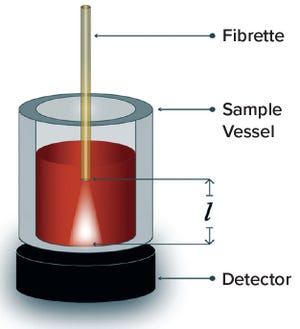
Figure 1: Mechanism of variable-pathlength UV-vis technology; l = the distance between the tip of the light-delivering fibrette and the inside bottom of the sample vessel.
Viral filtration (VF) using nanofilters removes endogenous and/or adventitious viruses from biologic drug-substance manufacturing processes (
1
). The gold particle test (GPT) is performed as part of postuse integrity testing — to complement postuse leakage testing — for cellulose filters such as Planova 20N filters from Asahi Kasei Corporation.
First, a proprietary gold-colloid solution matched to the filter type (e.g., 20N) is filtered through the test article. That filter’s pore-size distribution can be assessed using spectrophotometric absorbance readings of the integrity-test solution — e.g., 1:10 dilution of Asahi Gold Particle solution AGP-HA20 to test Planova 20N — and the correct filtrate sample (minimum volume collected for measuring absorbance) to calculate the gold-particle removal rate as a logarithmic reduction value (LRV)...
Figure 1: Adenovirus downstream process
Historically, companies developing vaccines have used attenuated pathogens, inactivated infectious agents, or antigenic constituents purified from pathogenic sources. In the past 20 years, technological advances such as recombination and viral vectors, have enabled development of vaccines against diseases with previously no available treatments (
1
).
Viral vectors have become one of the most rapidly evolving and promising fields in vaccinology and regenerative medicine. In addition to preventing infectious disease, they have a broad range of potential applications, including treatment of hereditary genetic disorders and cancers.
Adenoviruses are the most common vectors used to develop recombinant vaccines. They allow for development of vaccines against highly infectious and pathogenic viruses that would be too dangerous or technically difficult to produce at an industrial scale (
2
).
Vaccine developers modify the adenoviral vectors to express antigens from specifi...
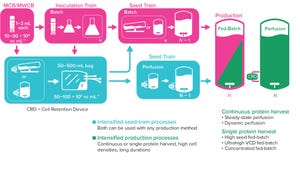
Figure 1: Perfusion can be used to increase viable cell density in the seed train to inoculate the production bioreactor with a higher cell density and in the production bioreactor.
As the need for novel therapeutics increases, so does pressure on the biopharmaceutical industry to improve productivity, accelerate development, increase, and reduce costs — all while ensuring drug product quality. Upstream intensification strategies such as perfusion culture can address those challenges and achieve higher protein titers that can translate into higher throughput, improved flexibility, and compressed timelines.
Successful implementation of perfusion culture or the transition to perfusion from fed-batch culture requires a different and strategic approach to media selection, not just for the bioreactor itself, but for clone selection, cryopreservation, and cell expansion. Here, we explore the foundational role of media across the upstream workflow, particularly for intensified processes, and how high cell densit...
Sartocheck 5 Plus filter integrity tester
Filter integrity is a fundamental element of sterility assurance during production of biopharmaceutical and vaccine products. Integrity test results are a key foundation for drug lot release, so any external element that could affect their reliability must be viewed as a critical issue. But when should a filter integrity test be performed?
This article highlights the Sartocheck 5 Plus filter integrity tester as a means to address regulatory requirements.
Please fill out the form below to read the full article now.
References
1
Antonsen HR, et al. Technical Report 26: Sterilizing Filtration of Liquids. PDA J. Pharm. Sci. Technol. 62(5S) 2008: 2–60.
2
EU Annex 1: Manufacture of Sterile Medicinal Products.
European Commission: Brussels, Belgium, November 2008; https://ec.europa.eu/health/sites/health/files/files/gmp/2017_12_pc_annex1_consultation_document.pdf.
3
ICH Q9: Quality Risk Management.
US Fed. Reg.
71(106) 2006: 32105–32106; https://www.ema.europa.e...
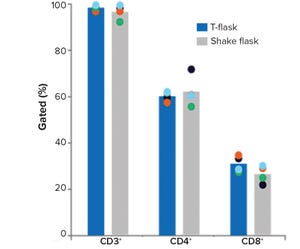
Figure 1: Comparison of small-scale culturing methods; phenotypic analysis of T cells and subpopulations CD4+ and CD8+ cells by flow cytometry at the end of culture; bars represent the average of four biological replicates with individual values shown by dots.
It is an exciting time to be involved in the cell and gene therapy industry. We have come to a tipping point where we have shown that the science works. Now we need to industrialize and standardize it, so we can get these life-saving therapies to more patients.
As an industry, we can learn from all the experience in the biologics space. With monoclonal antibodies (MAbs), we have transitioned from a manual process to a more standardized and automated set-up. We want to do the same thing for cell and gene therapies — but much faster.
We need to come to a standardized set-up and get to a closed and automated system. That will take us to a platform that will serve large patient populations while reducing both costs and risks. GE Healthcare Life Sciences...
Researchers traditionally have produced lentivirus (LV) in adherent cultures by transient transfection using media containing animal serum. The method is inexpensive for process development (PD) and early clinical trials, but it requires increased operator manipulations and costs during scale-up. Development scientist Adam McLeod delivered an Ask the Expert webinar on 27 August 2019 to illustrate how GE Healthcare Life Sciences at the Centre for Advanced Therapeutic Cell Technologies (CATCT) in Toronto, Canada, approaches lentiviral vector (LVV) manufacturing. McLeod explained that the team induced a LV producer cell line and then recovered LVVs by filtration. Upfront costs increase with that strategy, but McLeod says that the changes greatly simplify scale-up.
McLeod’s Presentation
For transient transfection processes, the goal is to scale cell culture development to manufacturing levels as quickly as possible. However, to reduce cost at larger scales and to decrease batch-to-batch variability, a LV prod...
Sponsored Content
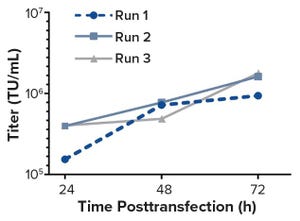
Figure 1: Functional titers (TU/mL) of the unconcentrated LV supernatant determined by using flow cytometric detection of GFP-positive cells
Although demand for lentiviral vectors (LVs) for cell and gene therapy is increasing, the standard two-dimensional culture systems used to produce LVs present significant disadvantages. Current bottlenecks in LV production are caused mainly by such disadvantages. Switching to use of bioreactors can eliminate those problems because bioreactors offer the benefits of process automation, tight regulation of production conditions, and reduced labor input. The study reported herein was carried out by the group of David Parsons at the University of Adelaide. It was one of the first attempts at transient LV production in a stirred-tank bioreactor with cells adherently grown on Fibra-Cel disks.
Just fill out the form below to read the full article now.
References
1
McCarron A, et al.
Transient Lentiviral Vector Production in HEK293TCells Using the BioFlo 320 Control Station...
Single-use systems (SUSs) are becoming increasingly common in bioprocessing operations because of their low capital requirements and validation costs. As this trend continues to develop, pharmaceutical manufacturers are asking SUS manufacturers to provide assurance that their products comply with current good manufacturing practices (CGMPs) and do not alter drug products by exceeding established operating ranges.
Certification of product cleanliness has become common for manufacturers of final packaging components such as vials and stoppers, but rarely are tubing products certified to meet endotoxin, bioburden, and particulate standards. In some cases, pharmaceutical manufacturers have taken their own risk-mitigation measures to reduce potential contamination, such as by rinsing tubing products with water for injection (WFI). However, such strategies are costly and, in some cases, unfeasible. Given the costs of this approach, the pharmaceutical manufacturing industry is primed for a change in the typical ...
Jan Van Hauwermeiren (vice president of sales and marketing at Ovizio Imaging Systems) joined BPI for an Ask the Expert webinar on 29 August 2019. He demonstrated how his company’s iLine F microscopy system combines sensitive optical capability with machine learning to monitor chimeric antigen receptor (CAR) T-cell expansion, transduction, and activation. The device captures holographic images of cells as they pass through a single-use conduit running between a microscope and bioreactor. Cells then funnel back into culture without additional handing. Because the device records 1,200 images per day, it precisely tracks individual cells over time. The system is fully automated and enclosed to ensure regulation-compliant analyses that do not inhibit cellular development.
Van Hauwermeiren’s Presentation
Each image captured by the iLine F system is a composite. When CAR T cells travel from a bioreactor through a gamma-irradiated BioConnect single-use channel, they cross a sterile probe that records data regard...
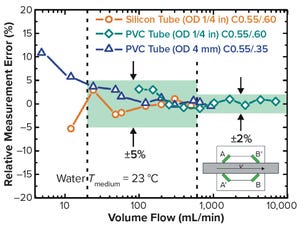
Figure 1: Measuring principle of the SONOFLOW CO.55 clamp-on flow sensors by operating transit-time difference method
Flow sensors placed at critical points in both upstream and downstream processes fulfill the regulatory goals of the process analytical technology (PAT) framework. PAT has been defined as a mechanism for design, analysis, and control of biotechnical and pharmaceutical manufacturing processes through measurement of critical process parameters (CPP). Constant flow monitoring can support its overall targets fundamentally to
Continuous processing is linked closely to the movement of fluids through a system and involves adjustment of volume and flow rates fed by peristaltic pumps. Noninvasive flow sensors such as SONOFLOW CO.55 sensors can fulfill the requirements of PAT reliably. Within upstream and downstream processes, clamp-on flowmeters have been placed at a number of critical points.
This BPI article explains how noninvasive flow sensors such as SONOFLOW CO.55 sensors can fulfill the requ...
Image: AdobeStock/tadamichi
Cell and gene therapy (CGT) companies are opening the door to a paradigm shift in medicine. They’re rapidly developing some of the most exciting new therapeutics we’ve seen in decades, delivering meaningful results for patients and changing lives for the better. In the latest RSA Talent Equity report (1), we focus on the CGT sector, looking at the profile of leadership teams that bring the most value to shareholders in this exciting area.
Growing Pressure in CGT
CGT companies face a unique set of challenges. They are subject to short time frames, putting company leaders under high pressure from day one and creating the need for a faster growth strategy dependent on people and money. Management teams need to be built faster and be more broadly based than in purely R&D-focused drug discovery enterprises. We analyzed the profiles of the people at the heart of these companies and how they’ve assembled winning teams that evolve and change over time according to available funding and...