With autumn beginning to assert itself, our days here in Oregon are cooler, and long-awaited rain is coming. From drought and wildfires to tropical storms and flooding around the globe, we hope that you and your loved ones are making it through this year’s extreme weather events unscathed. And we continue to wish you well through the continuing healthcare crisis.
Autumn is time for the annual BPI Conference (including its parallel cell and gene therapy track), held as a hybrid event this year. We look forward to highlighting some presentations in upcoming reports and through submitted manuscripts. Meanwhile, we face the task of creating our upcoming media kit — and in it, our editorial calendar for 2022.
The calendar always requires us to balance industry knowledge with a certain level of prognostication and informed guesswork. Will today’s “hot topics” still be high priority by next summer or fall? Which aspects of familiar topics have we yet to focus on (and who will write about them for us)? A primary ...
The number of collaboration arrangements for emerging technologies is increasing significantly, especially among companies developing COVID-19 diagnostics, vaccines, and therapeutics. Consequently, a massive amount of capital was invested in the biopharmaceutical industry in 2020. These deals range in value from the low millions to several billion dollars, representing significant risks and value for investors.
Some recipients of that capital are seeking partners with whom to collaborate, sharing both the costs and risks associated with their activities and bringing innovative technologies to market. Such collaborations take on different forms and typically involve joint development, licensing, and ultimate commercialization of intellectual property (IP) related to potential new drugs or medical devices. All parties share profits or losses.
With significant pressure to deliver for investors, valuable resources at stake, and increasing pressure to move quickly, companies might have little time to analyze a...
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline Q12 (
1
) (step 4 sign-off in November 2019) is in the process of being implemented in a number of regulatory regions. The document provides additional frameworks for pharmaceutical life-cycle management. It is intended to support globally harmonized regulatory tools such as established conditions (ECs) and product life cycle management (PLCM) documents to facilitate postapproval changes to chemistry, manufacturing, and controls (CMC). Although a harmonized framework and an agreed-upon plan for managing changes have the potential to be transformative, the practical implementation of the ICH Q12 concepts has yet to be fully realized. On 27 January 2020, a CASSS Sharing Science Solutions Working Group workshop titled “Established Conditions for Biopharmaceutical Products: Ion-Exchange Chromatography” was held in Washington, DC. The meeting brought together a small group of regulators and i...
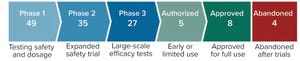
The COVID-19 pandemic is the greatest global health threat caused by a virus since the influenza pandemic of 1918 (and, before that, innumerable smallpox outbreaks throughout history). So far, the number of infections and deaths has not reached levels seen during the “Spanish flu” pandemic. However, travel, global trade, and modern factors such as misinformation on social media have increased infection rates and risks of infection. As of 15 July 2021, the World Health Organization (WHO) had reported 188,655,968 confirmed cases and 4,067,517 deaths worldwide (
1
).
Figure 1: Coronavirus vaccine tracker (Financial Times, 26 April 2021)
Throughout most of the world — particularly in the United States, Europe, and Japan — patents provide incentives for the development of vaccines, small-molecule and biologic antiviral drugs, and other drug products. Patents protect a developer’s investments of time and resources. The rapid development of COVID-19 vaccines is an example of the benefits that incentives patents ...
According to the founder and executive chairman of the World Economic Forum, Klaus Schwab, the fourth industrial revolution began in the 21st century and is characterized by an unprecedented development and exponential growth of a high-technology industry transforming society at every level (
1
–
4
). In particular, healthcare systems are evolving rapidly to adapt to the new reality. According to Forbes, the main technologies currently shifting the paradigm of medical research are artificial intelligence (AI) and machine learning (ML) (
5
), both defined in the “Terms and Abbreviations” box. From a marketing perspective, experts predict that the global AI healthcare market will grow from US$4.9 billion in 2020 to reach $45.2 billion by 2026 (
6
).
AI makes it possible to turn the challenge of big-data growth within the biomedical sector into an advantage. It is not surprising that application of AI already has been integrated into drug-discovery processes: 40% of drug-discovery start-ups already are explo...
Virus removal/inactivation is a major concern in the safety of monoclonal antibodies (MAbs) and other recombinant-protein drugs. Some methods (such as nanofiltration and low-pH inactivation) have been demonstrated repeatedly by the industry to be reliable for most viruses, with >4 log10 removal. Based on my company’s virus-removal experiences with its MAb downstream-process platform, we propose a “bracketing method” — testing only samples that lie at the extremes of a design space — to prove proactively that small differences in operating parameters among different product manufacturing processes do not affect the virus log10 reduction value (LRV). At our company, we determine LRV based on our experience and data from our virus removal/inactivation platform to ensure the viral safety of biopharmaceuticals in development.
At the investigational new drug (IND) stage of development, virus-safety studies are performed to demonstrate acceptable levels of safety for clinical trial materials (CTMs). Virus detect...
Figure 1:
A biologics manufacturing process consists of three main steps.
Continued process verification (CPV) is an integral part of process validation for the manufacture of human and animal drugs and biological products (
1
). It is designed to meet three primary goals: maintain a validated state of products, their processes, and related systems; enable continuous process improvements; and meet regulatory requirements for life-cycle validation.
A CPV program for a biologic product entails regular collection of data related to critical process parameters (CPPs) and critical quality attributes (CQAs) and the preprocessing, analysis, and evaluation of those data to ensure that a biomanufacturing process is in a state of control. Typically, CPV is performed at a univariate level in which each parameter or attribute associated with a process or unit operation is evaluated independently. That creates multiple univariate plots for review. In addition, it results in a loss of information related to the correl...
Typically a concern with cereal grains, mycotoxins are produced by filamentous fungi under specific conditions, as shown here on damp buckwheat. Animals that consume contaminated feed can present detectable mycotoxin levels in their milk (
https://www.istockphoto.com
)
Sevenfact eptacog beta is a new recombinant human factor VIIa (rFVIIa) developed by LFB SA in Les Ulis, France, as a bypassing agent (BPA) for treatment and control of bleeding in people with hemophilia A and B and inhibitors (
1
,
2
). The product was approved for use in adults and adolescents by the US Food and Drug Administration (FDA) in April 2020 (
3
). It is expressed in the milk of transgenic rabbits and purified through a multistep process using both filtration and chromatography. The source material (transgenic rabbit milk) naturally contains a microbiological load. To manage that, the collection process is controlled carefully, resulting in a historical average bioburden of 1,800 CFU/mL (
n
= 116). That is well below the prepas...
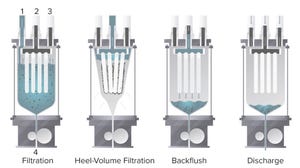
Figure 1:
The four main steps of a Contibac SU filtration cycle (as defined in text, below)
Over the past few decades, single-use (SU) technology has increased bioproduction efficiency significantly, especially with the introduction of disposable bioreactors in upstream processing. To keep pace with major developments and increases in upstream capacity, downstream processes also must increase capacity and efficiency. However, cell harvesting and downstream processing continue to present bottlenecks in manufacturing (
1
).
Typical clarification processes are composed of primary and secondary clarification steps, such as centrifugation followed by depth filtration, respectively (
1
). Two sets of SU depth filters can be used for primary and secondary clarification, although they tend to foul quickly and must be replaced. Here we introduce the new Contibac SU filtration technology (Dr. Mueller AG, DrM) that combines primary and secondary steps in one unit operation. We compare that to an existing and well-k...
Corning CellSTACK cell culture chambers can help deliver more yield with less labor, especially when configured as closed systems.
As viral vectors continue to push gene therapy innovations closer to market, many researchers are setting their sights on optimizing transfection, the process of delivering corrective genetic material into cells. It’s not just a question of how to transfect them, but also how to do so efficiently and at high volumes. Approaches that work for one cell line might not perform well for others, and transfection protocols can have different implications for scalability and cost during production for clinical trials.
For those and other reasons, experts usually reply, “It depends,” when asked about the best transfection strategies for gene therapy programs based on adenoassociated virus (AAV). As a senior bioprocess application scientist at Corning Life Sciences, Ann Rossi Bilodeau often receives questions from researchers about which approaches they should apply: Should they use sus...
Cell and gene therapies (CGTs) are positioned currently as last-chance, “miracle” cures for patients who have severe illnesses. Such promises require innovation. Despite the cutting-edge science and significant investment that goes into CGT development, fundamental challenges remain, including patient access. The highly personalized nature of autologous-therapy development presents myriad logistical, financial, and manufacturing challenges to ensuring global access to treatment. Understanding a patient’s journey to treatment is vitally important to achieving that goal.
Barriers to Cell and Gene Therapy Access
Awareness:
At Terumo Blood and Cell Technologies, we’ve learned that educating patients and clinicians about available technologies and therapeutic approaches — such as treatment of sickle cell disease using automated red blood cell exchange — can broaden access to treatment. Even if a cure existed for the condition, many sickle cell patients in the United States would go untreated simply because th...
Recombinant proteins such as growth factors and cytokines are essential for cell therapy, gene therapy and regenerative medicine research, development and manufacturing. These proteins are critical in the production of desired cell types and subsequent differentiation of cells, to deliver the desired effect.
Founded 15 years ago, Shenandoah Biotechnology applies a proprietary method of folding and purifying recombinant proteins from both bacterial and mammalian systems to enable cost-effective, large-scale production of Cell Therapy Grade proteins to support these groundbreaking treatments. The company’s product portfolio includes human interleukins such as IL-2, IL-7, IL-15, and IL-21 as well as TGF-β1, FGF2, and other growth factors to support the ex vivo culture of cells.
In this article, BPI speaks with Pamela De Lacy, president of Shenandoah about the benefits offered by their approach to production of these proteins and their new 15,000-ft
2
current good manufacturing practice (CGMP) production fac...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.