September 2018
Launched in June 2018, the
BioProcess Insider
digital information portal delivers the latest financial and business news and expert insider views influencing the commercialization of biopharmaceuticals. Here are just a few recent stories edited for our space limitations in print. For more discussion and in-depth analysis, check out the website at
www.bioprocessinsider.com
. Every edition provides expert and insider perspectives on current financial movements and deal-making; the newest technology purchases and capacity investments; regulations affecting the bioprocessing sector; global market actions and reactions; and industry trends.
HyperStack Shortage Caused By Gene Therapy Surge
Adoption of viral-vector technology in cell and gene therapy has led to six-month waiting lists for multilayer culture vessels. HyperStack vendor Corning plans to more than double its manufacturing capacity in response. Multilayer, scalable, and single-use culture flasks are used to produce viral vectors and gene therapies...
WWW.COPPERHOUNDPICTURES.COM
Magazine editors live in a strange warped time frame. We put the finishing touches on this very September issue in mid-August, having begun working with the materials a month before that. Meanwhile, we’re in talks with authors and companies who will be contributing to the pages of our October and November–December issues and inserts. Anne is working already to acquire manuscripts for the early issues of 2019. When it comes to eBooks, however, we actually put them together during the month that they come out — having cut the printing “middle man” out of the process. But we just finished working together with our publisher to determine what our entire eBook schedule will include next year.
You can imagine, then, that when I saw the movie
Interstellar
, it wasn’t all that difficult to for me to wrap my head around the relativity-induced time warps involved. This year, my husband and I are raising a new Labrador puppy, and I think our human instincts are a bit confused by how fast...
WWW.ISTOCKPHOTO.COM
The primary goal of container–closure integrity (CCI) is to maintain the sterility and product quality of parenteral biopharmaceuticals throughout their shelf life and use. Guidelines detailing the initial CCI qualification and validation requirements have been defined and can be found in the
US Pharmacopeia
chapter 1207 (USP<1207>) (
1
). The guidelines described in USP<1207> can be applied to any common CCI testing (CCIT) method to achieve a method suited for its intended use within a drug product lifecycle. CCI is not a single time-point event, but rather an integral and holistic process. It is evaluated and stressed throughout the manufacturing lifecycle of a sterile drug product (e.g., during primary package development, line qualifications, product manufacturing qualifications, stability testing, change-control process, and shipping studies) and tested when required.
Within the pharmaceutical industry, the CCIT technology landscape is changing rapidly, and new technologies are ...
ADOBE STOCK (HTTPS://STOCK.ADOBE.COM), FREE IMAGES (WWW.FREEIMAGES.COM), AND CHERYL SCOTT
While the growth in biopharmaceutical manufacturing capacity in developed, major market countries is continuing its slow and steady climb, developing regions often are seeing double that growth rate. Over the past eight years, as detailed in the “About the Data” box, our company’s index of the top 1,000 biomanufacturing facilities (
1
) has tracked and ranked bioprocessing facilities worldwide in terms of
Significant strategic implications come with those changes in growth rates, especially in developing regions. As production of biologics becomes more globalized, we are regularly seeing active pharmaceutical ingredients (APIs) produced in one region, fill–finish activities performed in another, and marketing and sales done in yet another. As more opportunities develop outside the US/EU markets, both biologics innovators and their suppliers are considering how these shifts in growth require predictive plans.
Our data...
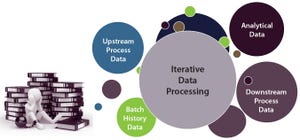
Managing large amounts of data presents biopharmaceutical companies of all sizes with the need to adopt more efficient ways to handle the ongoing influx of information. At KNect365’s September 2017 Cell and Gene Therapy conference in Boston, Lisa Graham (founder and chief executive officer of Alkemy Innovation, Inc. in Bend, OR) spoke about the need for data management, data analysis, and process monitoring systems to evolve. Although she was speaking at a cell therapy event, her points are applicable to the biopharmaceutical industry at large — which can learn a great deal overall from data management practices in other industries.
Alkemy Innovation is an engineering company delivering science-based solutions in business/engineering intelligence and strategic data acquisition and process analytical technology (PAT). In a recent eBook for BPI, Graham examined how different software and hardware applications can provide required scientific insight for success with bioprocessing in two industries: brewing a...
JUPITERIMAGES (WWW.GETTYIMAGES.COM)
Each year, over 20% of the human population is infected with the influenza virus, resulting in 250,000–500,000 related deaths globally and ~38,000 deaths in the United States alone. Of further concern is the potential for pandemic outbreaks, which pose a severe worldwide threat to public health (
1
,
2
). Vaccination has proven to be a critical tool for controlling the spread of infectious diseases, as evidenced by the eradication of polio, smallpox, and diphtheria in most parts of the world.
Influenza presents a unique challenge compared with those viruses: Two main viral-surface glycoproteins — hemagglutinin (HA) and neuraminidase (NA) — cause an immune response and the resulting flu-like symptoms. But they experience antigenic drift and shift that enable new mutant virions to evade detection by host antibodies. Thus, flu pandemics can reoccur. Although vaccination is the most effective means of preventing influenza infection, new vaccines must be created each year t...
WWW.ISTOCKPHOTO.COM
Before a pharmaceutical product is introduced into humans, either in a clinical trial or as a marketed product, virus safety must be evaluated carefully. Virus safety normally is ensured using a
three step complementary approach: selecting and testing cell lines and/or raw materials for the absence of viruses, testing the product at appropriate steps of production, and assessing the capacity of a production process to clear infectious viruses (
1
). The latter (also referred to as
viral clearance
) is the subject herein. Spiking studies are conducted to evaluate the capacity of a purification process to clear viruses. Steps that are expected to clear viruses are studied with down-scaled laboratory experiments, in which different types of viruses are introduced deliberately and virus content before and after the step is evaluated. Clearance of a virus is expressed as a reduction factor (RF) calculated as the total virus before a virus clearance step divided by the total virus after the...
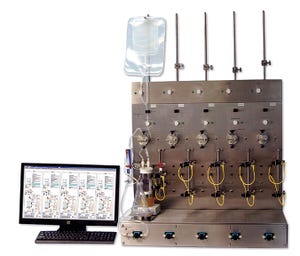
Photo 1: Five-station tangential-flow filtration (TFF) screening system design agreed upon by the collaborators
Ultrafiltration and diafiltration (UF–DF) of therapeutic proteins are performed in either tangential or crossflow mode using membrane filters. UF–DF plays a critical role in both downstream and upstream processes for the biopharmaceutical industry (
1
). In upstream production processes, classical tangential-flow filtration (TFF) or alternating tangential-flow (ATF) systems are used in high–cell-density perfusion for protein expression by cell culture (
2
). TFF is used in downstream processing for UF–DF and concentration of therapeutic proteins. TFF unit operations are common in protein purification because of their scalability and amenability to continuous processing (
3
–
5
). Typical TFF process development involves optimization of transmembrane pressure (TMP), permeate flux, feed flowrate, buffer compositions, and the interdependence of all those parameters. Optimization requires a broadly ...
Figure 1: Increasing product shelf life; postthaw stability at 2–8 °C (study carried out in collaboration with the University of Barcelona and Xcelia of Banc de Sang Teixits SA)
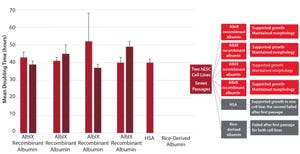
Stem cell therapies are some of the most cutting-edge and sophisticated therapeutic developments. They offer an attractive alternative approach to more widely used treatments for conditions such as multiple sclerosis, metabolic diseases, cardiovascular disease, liver disease, and cancer. But developers still face challenges, some of which can be addressed by the use of recombinant human albumin. As a long-established
ingredient of cell culture media, albumin is well recognized for its ability to facilitate growth of many cell types. The industry is expanding its use of high-quality, fully recombinant, current good manufacturing practice (CGMP) excipients in cryopreservation and formulation of stem cell therapies. Herein we describe the manufacturing, formulating, and handling challenges associated with the development of cell ther...
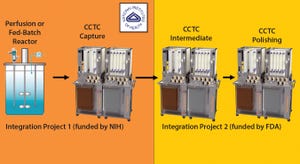
Figure 1: PendoTECH turbidity flowcell placed at the resin outlet of the CCTC; resin passing through the flowcell indicates a signal breakthrough, and the system is set to recirculation once the resin in the system has reached steady state. The steady-state “peak-free”product stream can be measured in-line with PendoTECH UV flowcells.
A strong connection between turbidity and total suspended solids (TSS) has been linked in the past to measuring well defined particles in processes. Optical density probes have seen wide adoption in the biotechnology industry for monitoring cell growth within a bioreactor, whereas in-line turbidity sensors have been used to monitor filter performance. Turbidity measurements offer a rapid quantification of suspended
solids but have not been used in the biotechnology industry for chromatographic resins. In this study, turbidity measured with equipment developed by PendoTECH was used with novel continuous chromatography technology developed by Chromatan for
accurate measurement...
Figure 1: Features of the Thermo Fisher HyPerforma 2,000-L single-use bioreactor
As the biopharmaceutical industry continues toward streamlined bioprocessing and intensified cell-culture biology, selection criteria of single-use bioreactors (S.U.B.s) and other bioprocessing technologies will become increasingly rigorous, emphasizing the importance of considering every aspect of technologies under evaluation. In part 1, we discussed performance for process control, including the maintenance of critical process parameters (CPPs), and highlighted bioreactor performance (e.g., mass transfer, power per volume, and temperature control) as a critical consideration during the selection of S.U.B.s (
1
). Part 2 focuses on physical and operational aspects of Thermo Fisher S.U.B.s, including hardware ergonomics, bioprocessing container flexibility, and quality vendor relationships.
S.U.B. Hardware
Hardware (e.g., shell, skid, control tower, and human–machine interface, HMI) is a key element in dictating the usabilit...
The blockbuster business model may have paid off in the past, but tomorrow’s biopharmaceutical successes will depend more on rapid and diverse discovery than on any single breakthrough. In the race to get new therapies from research and development (R&D) into pharmacies, next-generation laboratory space could become a game-changer.
Blockbuster drugs typically were made in industrial laboratories — and industrial-strength measures were required to reconfigure those spaces as new research priorities emerged over time. Facing changing patient needs and ongoing financial pressures, biotechnology companies don’t have the luxury of time or resources to undergo major laboratory overhauls.
This presents a clear case for taking a different approach to laboratory design. To maintain (or improve) their profit margins, most organizations must speed up R&D timelines dramatically. The average return on such investments for large biopharmaceutical companies dropped from 10.1% in 2010 to 3.2% in 2017, according to Deloit...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.

schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)