GE Healthcare 2017 Special Report
As the need to accelerate biopharmaceutical development around the world continues to grow, biomanufacturers face a host of challenges so they, too, can grow. Increasing process productivity, reducing cost, mitigating risk, and bringing products to market faster are just a few of the issues frequently addressed. But with support in process development, cGMP manufacturing and training, accelerating bioprocess development can become less challenging.
Several biomanufacturers have successfully navigated these issues in collaboration with GE Healthcare’s Fast Trak Services. Whether it was through process and analytical development, process scale-up, or manufacturing of drug material for use in toxicology studies, recent collaborations have yielded remarkable results. The following case studies describe projects in which collaborative efforts resulted in fast resolutions to common biomanufacturing challenges.
Pfizer in China
Working with a service provider is one strategy that biosimilar manufacturers can purs...
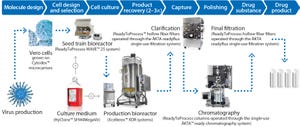
Figure 1: Process train comprises single-use equipment from GE Healthcare that help accelerate flavivirus vaccine manufacturing. Included systems are suitable for biomanufacturing of regulated products under various quality management systems. The systems are controlled through either GE Healthcare’s UNICORN™ or Schneider Electric’s Wonderware® system control software. To enable use of the systems in regulated environments, both programs are configured for use in a 21 CFR Part 11 and GAMP 5 compliant manner. All records are stored in a single, unalterable database, including results and extended run documentation. Specially trained and certified engineers perform onsite IQ/OQs and CCPs in accordance with cGMP, as well as provide on-site training for relevant personnel.
As with all viral vaccines, the complex nature of flaviviruses makes process development technically challenging. In addition, vaccine production can be both costly and difficult to scale to meet market demands. In egg-based vaccine product...
Figure 1: Process flows for 10-L and 200-L upstream processes using single-use bioreactors from GE
In their pursuit of developing therapeutics, biopharmaceutical companies face many challenges, including pressure to meet aggressive timelines, reduce risk and cost, and increase speed to market. Addressing these challenges is especially critical for biosimilar manufacturers, who face fierce competition and an expanding footprint in emerging markets (
1
). The global biosimilars market is growing rapidly and is expected to exceed more than US$6 billion by 2020 (
2
).
This case study describes how Pfizer is collaborating with GE Healthcare to achieve regulatory approval of biosimilars in China by streamlining process transfer strategies and implementing both single-use technologies and flexible, deployable manufacturing solutions.
Customer Needs and Concerns
Pfizer was interested in collaborating with a service provider to reduce risk and increase the speed to market of two biosimilar monoclonal antibodies (M...
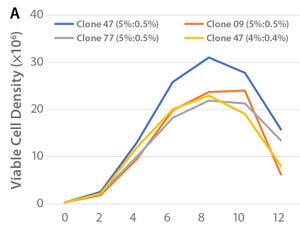
Figure 1: (A) Viable cell density over the culture period and (B) cell productivity on Day 12 of the tested clones in shakeflask cultures
Although the demographic of orphan therapies is small, making therapies for rare diseases available has a huge impact for the affected patients. Cooperation to expand capacity and expertise during process development and manufacturing for preclinical and clinical phase studies is one way to increase speed to market. This case study shares the work of GE’s Fast Trak Services team to help accelerate development of a process for cGMP production of material for toxicology studies.
Frequent communication between the Fast Trak team and the client ensured transparency while protecting customer’s intellectual properties. GE scientists worked closely with Roivant Sciences to facilitate tech transfer, and a cGMP manufacturing process was developed. As a result, 400 grams of RVT-801 was produced for toxicology studies in 16 months.
Background
Acid ceramidase is coded by the ASAH1...
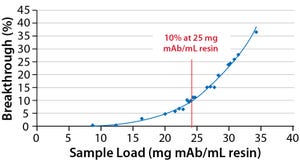
Figure 1: Results from testing of DBC of MabSelect SuRe resin
Biosimilars represent an innovative solution that can benefit both patients and healthcare systems by reducing the burden of rising treatment costs. To improve the availability, price, and access of medicines, many countries are implementing strategies to establish their own production capacity. To support such development, MAbxience (a Spanish biotechnology company specialized in research, development, and manufacture of biosimilar drugs) is committed to provide the manufacturing of high-quality products and processes that meet regulatory and technical requirements in all countries where it operates, using cutting edge single-use technology. Currently, MAbxience has sales contracts in more than 70 countries.
One of the biosimilar specialties present in MAbxience pipeline constitutes an Fc-fusion protein, with a molecular weight of Mr 150,000 and an isoelectric point of <5, for which a first-generation process was established by a third-party c...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.