Sartorius Stedim Special Report 2017
Single-use technologies are now dominant for the clinical production of biopharmaceuticals and are becoming more mainstream within commercial manufacturing facilities. They allow biologics manufacturers to decrease the footprint of their facilities by approximately 20% because of a reduced need for utilities that generate water, steam, and clean-in-place solutions. Engineers believe that the capital outlay for a single-use facility is 25–45% less than for a facility based on stainless steel equipment. Similarly, they estimate that such facilities need half the water and energy during operations and can be constructed in as little as 18 months. That is in contrast to the three years it can take to get a stainless steel facility up and running. Furthermore, the risk of product cross-contamination between batches is considerably reduced.
Biomanufacturers must be able to trust single-use technology as they implement it into increasingly critical bioprocessing process steps and applications. We have seen evide...
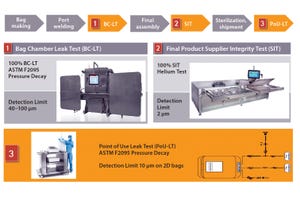
Failures in the integrity of single-use systems during commercial manufacturing can cause a number of serious problems for biomanufacturers. A loss of system integrity during processing can allow environmental contaminants that can be dangerous for patients (e.g., microbes) to enter a process. Biopharmaceuticals and their intermediates can be highly potent or even infectious agents, so an integrity failure can jeopardize the safety of operators. In severe cases when biomanufacturers cannot ensure the quality of drug products for fear of a contamination, the supply of life-saving medicines to patients can be restricted. Finally, significant costs can be associated with product losses and quality investigations that arise because of leaks within systems.
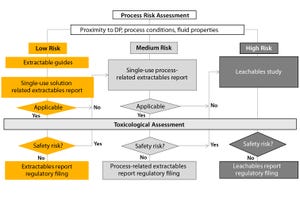
It is perhaps unsurprising that single-use container–closures are coming under increasing scrutiny from regulators. At many industry meetings, participants from suppliers, end users, and regulators discuss the topic.
For example, the US Food and Drug Admini...
The pipeline of biopharmaceuticals remains strong, and the market for biologics could exceed US$450 billion by 2025. Analysts predict that sales within segments such as regenerative medicine and antibody–drug conjugates (ADCs) will grow faster than 20% each year. Yet considerable challenges remain for biopharmaceutical companies to overcome if they wish to be successful. First, they must reach the market quickly. Analysis by the Boston Consulting Group shows that the proportion of available value that a newly launched product can capture is a function of both the therapeutic advantage that it provides and its position in the launch of competing products. Being first to market is critical for commercial success of a new biologic.
Commercial risks inherent in developing new biological products have not gone away. Only a small proportion of such products that enter clinical assessment will make it through to a commercial launch. Although speed to market is important, companies must be careful to balance that...
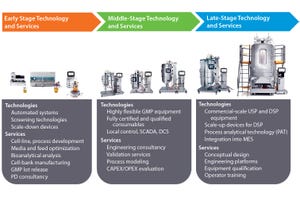
Biopharmaceutical companies can develop entire end-to-end single-use production platforms and use them for commercial manufacture of their biological products. Single-use facilities are flexible, can be implemented quickly, and do not require the large up-front capital investments needed for stainless steel equivalents. However, single-use facilities must be supplied with a large quantity of high-quality consumables. Biomanufacturers should pay close attention to their supply chains for those consumables to ensure that they are robust, integral, and fully compatible with biological expression systems and products.
Simply attempting to ensure that single-use manufacturing platforms provide equivalent performance to stainless steel facilities is a missed opportunity. Innovations in the fields of process analytical technologies (PAT) and data analytics allow engineers to gain greater understanding and control of single-use processes. Those innovations deliver the combined benefits of more consistent producti...
The preceding articles have shown how biopharmaceutical companies increasingly are adopting single-use manufacturing technologies for commercial production facilities as their confidence grows in the material science, supply chains, and robustness of single-use systems. Single-use suppliers can support adoption of end-to-end process platforms in commercial manufacturing settings with dedicated teams of experts in process development, engineering, and regulatory support. Advances in process analytical technology (PAT) are providing engineers with greater information on conditions within their single-use bioprocess platforms to allow for increasing levels of control.
Engineers can define set points for important parameters such as pH, temperature, and dissolved oxygen to provide consistent operation, use PAT tools to measure necessary parameters such as glucose and cell density, and then use regulatory controllers to maintain those parameters at the predefined set points. Some problems come with using this ...
The development and launch of new biopharmaceutical products is a very challenging and risky process. Sponsors must get their products into clinical testing as quickly as possible to beat their competition and take the greatest share of the market. Yet that need for speed must not lead to companies launching products with inefficient manufacturing processes that ultimately will leave them vulnerable to attack from low-cost competition. Single-use systems have been a significant enabling technology for companies developing new biologics because of their inherent flexibility and low upfront costs, which help mitigate financial risk during process development.
As the technology has matured, more companies are becoming interested in widespread adoption of single-use systems even in commercial production settings. That would allow them to take advantage of the same flexibility of single-use technology while creating agile manufacturing networks that can respond to variations in demand, readily produce multiple...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.