While editing and proofreading an issue, we often see statements that suggest other intriguing angles to explore. But deadlines loom, and our notes to follow up on those ideas can get lost in the shuffle. And because we pursue a good portion of our manuscripts (cue image of editor chasing after conference speakers in crowded hallways), those notes have value. Seemingly casual statements in talks and manuscripts can help us focus our acquisition efforts.
Working through those mental “loops,” adding incremental bits of insight in hopes of an
aha
moment can be frustrating. “But what’s new?” Debates continue over single-use, stainless-steel, and hybrid systems; continuous and batch processing; and artificial intelligence (AI), digitalization, and iterations of “4.0.” Questions arise about risk mitigation, product variation, and process platforms; the industry’s resistance to change and regulators’ comfort with new technologies; differences in available resources and capabilities between small and large comp...
HTTPS://STOCK.ADOBE.COM
In the April 2023 issue of BPI, Leonardo Ferreira discussed the biology of type 1 diabetes and how he has worked toward developing a cure for that disease at his laboratory at the Medical University of South Carolina using chimeric antigen receptor (CAR) T regulatory cells (Tregs) (
1
). He spoke about industry-wide advances in developing Treg technology and how lessons learned during preclinical trials can be applied in human trials. He also discussed what the industry needs to develop Treg advanced therapies successfully. We conclude our discussion here in Part 2.
The Advantages of Tregs
Why is your team researching advanced therapies based on CAR Tregs instead of CAR-bearing T effector cells or even monoclonal antibodies (MAbs)? How would Tregs treat type 1 diabetes?
There is a lot of evidence supporting CAR T cells for advanced therapies. The US Food and Drug Administration (FDA) has approved several CAR T-cell therapies — for example, CD19 CAR T cells and B-cell maturation an...
HTTPS://STOCK.ADOBE.COM
Developing novel medicinal products involves many processes and requires a number of different technologies and areas of expertise. Although large pharmaceutical companies can support in-house development and fill gaps by hiring contract development and manufacturing organizations (CDMOs), the cost, complexity, and scope of development are prohibitive for most academic or small- and medium-sized enterprises (SMEs). Such difficulties are amplified for vaccine developers, especially those working to treat vulnerable populations in low- and middle-income countries where profit potential is limited. A permanent vaccine research infrastructure (RI) in Europe could relieve developmental obstacles (
1
).
The COVID-19 pandemic highlighted the need for flexible vaccine-production capacity and operational excellence. Research innovations from university and SME professionals demonstrated the potential of mRNA vaccines (
2
). But COVID-19 mRNA vaccine successes have yet to translate to other ...
HTTPS://STOCK.ADOBE.COM
On average it can take or even exceed US$1 billion to get a pharmaceutical product to market, and nine out of 10 products developed never make it to commercialization (
1
). As technology advances, more potential therapies and preventatives are being developed and optimized by virtual companies. They are typically small, newly formed organizations that build their momentum on programs for novel products. Because many of the program activities are outsourced, virtual start-up companies developing pharmaceutical products raise concerns about ensuring product quality and regulatory compliance. Below, we analyze attributes to phase-appropriate quality assurance programs during research and development (R&D) into clinical studies. We focus on quality requirements needed to manufacture a compliant product at the appropriate phase of development for that product. Finally, a few case studies show how quality assurance has supported virtual and/or start-up companies.
Starting Points
Develop...
HTTPS://ADOBE.STOCK.COM
With digital innovations revolutionizing consumer-facing products such as medical devices, questions are arising about whether the biopharmaceutical and broader pharmaceutical industries are embracing digital transformation to drive process improvements and meet changing product demands. Below, Fausto Artico (global head and product director of innovation and data science at GSK) shares his insights about digitalization among pharmaceutical companies that are developing protein-based biologics, vaccines, and advanced therapies.
Artico has driven several of GSK’s digitalization initiatives, including work with artificial intelligence (AI) and machine learning (ML), cybersecurity, and “hyperautomation.” Before joining GSK, he worked on key digitalization and supercomputing projects at the NVIDIA Corporation in Santa Clara, CA, and at the IBM Thomas J. Watson Center in Yorktown Heights, NY. He holds doctoral degrees in information science and computer science from the University of Ca...
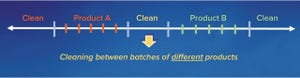
Cleaning Validation Acceptance Limits for Biological Process Residues: Part 1 — Acceptable Exposure of Degraded Proteins Based on Reference ImmunogensCleaning Validation Acceptance Limits for Biological Process Residues: Part 1 — Acceptable Exposure of Degraded Proteins Based on Reference Immunogens
Over the past decade, human therapeutic proteins (HTPs) have become far more potent, and consequently, their acceptable exposures have decreased substantially. That has led to commensurately lower acceptance limits for biological process residues. Simultaneously, host-cell and other protein concentrations have increased considerably, thereby making process equipment potentially more difficult to clean. These trends have made biopharmaceutical cleaning validation more challenging.
For example, the acceptance limits for many HTPs — based on acceptable exposures of active proteins — are on the order of 0.1 µg/cm
2
, which is an order of magnitude lower than the capability of most cleaning processes and analytical methods (
1
). As a result, acceptance limits for active protein residues often are not achievable. Thus, cleanability and cross-contamination are important regulatory considerations in biopharmaceutical manufacturing.
The degradation of HTPs during cleaning and sanitization provides a practical and...
HTTPS://WWW.ALAMY.COM
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidance on the testing and evaluation of viral safety of biotechnological products derived from characterized cell lines of human or animal origin through its harmonized guideline ICH Q5A (
1
). The latest revision, released for consultation in October 2022, maintains the key principles of previous versions while introducing key changes in response to important advances in the field. Those advances are covered in new sections that reflect improvements to current scientific knowledge. They include new product types that are amenable to viral clearance, including viral-vector–derived products; considerations for continuous manufacturing; and the use of prior knowledge (PrK) for modular validation and resin-reuse studies. Also discussed are new molecular analytical methods, particularly next-generation sequencing (NGS). The aims of this update are to reduce the need fo...
HTTPS://STOCK.ADOBE.COM
The 2011 process validation (PV) guidance document from the US Food and Drug Administration (FDA) states that the number of samples used for PV “should be adequate to provide sufficient statistical confidence of quality both within a batch and between batches. The confidence level selected can be based on risk analysis as it relates to the particular attribute under examination” (
1
). In alignment with those expectations, I present herein two statistical methodologies for calculating the necessary number of process performance qualification (PPQ) runs: the tolerance interval (TI) method and the process performance capability (PpK) method. Both entail the following sequence of steps for demonstrating that the PPQ results obtained have an acceptable statistical confidence:
1: Assess the risk of an attribute/parameter to be monitored.
2: Assess statistically the reliability for the sample size of data used to gain process knowledge.
3: Define targeted statistical confidence and relia...
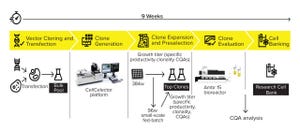
Speed to market is a critical business driver in the biopharmaceutical industry. However, drug development success also requires building a robust process that maximizes efficiency while limiting the cost of goods. Achieving time and cost savings without compromising product quality is critical. Development of a productive and stable cell line is the foundation of an efficient and high-performing bioprocess.
Cell-line development (CLD) represents some of the most resource-intensive steps within a process development pipeline. Bioprocess scientists must find a balance between reducing timelines and costs while still carrying out comprehensive CLD activities to ensure the desired level of performance. Early investment in technologies and services to accelerate the search for a high-performing cell line can pay dividends later (
1
).
Novel automated and high-throughput single-cell cloning technologies are being introduced into CLD processes to meet those business needs (
2
). For example, accelerated single-...
Monoclonal antibodies (MAbs) are the fastest-growing modality in clinical trials and more than 100 such therapies have been approved to treat diseases. But despite the modality’s success, MAb manufacturing is often contaminated by host-cell protein (HCP) by-products. Tom Valorose, senior product manager at Astrea Bioseparations, discussed how companies can reduce HCPs dramatically during MAb purification.
Valorose’s Presentation
Removing HCPs is an important part of MAb downstream processing. After cell harvest, scientists perform a primary capture using affinity chromatography with protein A or L resin. Anion-exchange chromatography then removes the majority of contaminating HCPs. However, at large batch sizes and process scales, the differing salt conditions required for protein elution and ion exchange necessitate a potentially disruptive dilution step.
Additionally, removing high– molecular-weight (HMW) aggregates requires hydrophobic-interaction chromatography (HIC), which separates molecules based o...
Biologic medicines have brought extraordinary clinical benefits to patients living with difficult conditions such as cancer and autoimmune and ophthalmic diseases. But such therapies are expensive, accounting for about 43% of pharmaceutical spending in the United States (
1
).
The Biologics Price Competition and Innovation Act of 2009 (BPCIA) created a regulatory pathway for a class of biologics called
biosimilars
, which replicate branded biologic drugs at lower cost (
2, 3
). The BPCIA was designed to provide greater access to biologics by bringing biosimilars to market, in turn stimulating competition and lowering the overall cost of healthcare.
Biosimilars first became available in the United States in 2015. Since their introduction, uptake patterns have differed across therapeutic areas. For instance, infliximab biosimilars began receiving regulatory approval in 2016, but they are used less frequently than is the reference product. However, in oncology, trastuzumab biosimilars have captured over 80%...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.