Development of a single drug, whether it is a new chemical entity, biotherapeutic, or cellular therapy, requires significant investment of resources. Care must be taken to choose analytical methods that are fit for the purpose of monitoring products and contaminants in the process. With expertise in labeling and detection, Enzo offers solutions to help rapidly analyze protein stability and integrity for all your bioprocessing needs.
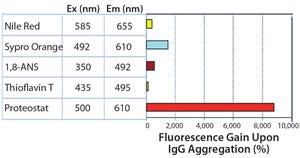
Figure 1: Broadest detection range from visible to subvisible particles
Protein Aggregation
The PROTEOSTAT® Protein Aggregation Assay (ENZ-51023) is a sensitive, homogeneous, fluorescence-based detection assay. The novel molecular rotor dye detects a broad range of different protein aggregates from visible to subvisible particles (Figure 1).
Figure 2A: Detects low % aggregate in concentrated samples
With convenient options for analysis, this high-throughput assay can be used on either a fluorescent microplate reader or flow cytometer. It provides a convenient, complementary ...
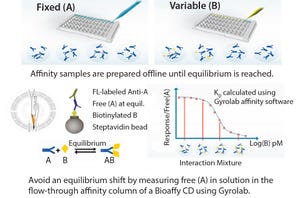
In the development of biotherapeutics, there is a need to quantitate antibody titer and to characterize antibody affinity against the target of interest. Such analyses are often challenged by the need for sensitivity, reproducibility, and higher throughput. Gyrolab systems automate and miniaturize affinity measurements and IgG titer quantification in a single platform using only nanoliter volumes with rapid time to results.
Figure 1: Gyrolab huIgG titer kits provide reliable measurement of human IgG titer over a broad dynamic range and with excellent reproducibility.
IgG Titer Immunoassays with Broader Ranges for Cell Line Development
The immunoassays used to determine IgG titer must have the flexibility to support a broad range of concentrations encountered from expression to production. Gyros recently launched ready-to-use kits to rapidly measure human IgG titer at a higher throughput using only nanoliter volumes for use on Gyrolab systems. The convenience and robustness of kits combined with the auto...
Host cell protein (HCP) levels in biologics are on the critical path for assessing product quality, and they pose a serious risk to drug safety. The challenge is to accurately quantify a complex mixture of HCP impurities, which vary in properties and abundance depending on cell line, media, and process parameters. HCP immunoassay analysis is based on polyclonal antibodies raised against HCPs from nontransfected cell lines.
Automated immunoassays with high-throughput (Gyrolab xP workstation, left) or single-CD configuration (Gyrolab xPlore, right)
How well a particular HCP assay recognizes all proteins depends on how well the antibodies match the HCP composition, their abundance, and their affinities. To cover the diversity of HCP mixtures from different bioprocesses, Gyros has developed a panel of five Gyrolab CHO-HCP kits to increase the possibility of finding the assay that best matches a specific CHO-K1 process. A scouting kit simplifies selection of the optimal generic kit with broader dynamic ranges,...
Assessment of thermal stability parameters of biologics is an integral part of biopharmaceutical research. The ever-growing number of biologics in development pipelines worldwide demands rapid and precise methods to quickly screen large sets of conditions in an easy and straight-forward manner.
In a recent study, we compared two methods for detection of thermal unfolding transition temperatures (
T
m
) of a therapeutic monoclonal antibody (MAb): nanoDSF, which analyzes changes in the fluorescence emission properties of proteins, and differential scanning calorimetry (µDSC), which detects changes in the heat capacity of a protein solution upon unfolding. Both nanoDSF and µDSC provide precise and consistent data. But nanoDSF also overcomes several limitations of µDSC (e.g., low throughput and high sample consumption) and thus represents the ideal technology for rapid and precise thermal stability screening in biopharmaceutical development.
Prometheus NT.Plex nanoDSF instrument
In a second study we demonstra...
Standardizing equipment in biopharmaceutical production streamlines operations, saving end users time and money. An area ripe for standardization is the use of genderless connections, those in which the connector halves are identical in design. Historically, single-use connections have involved male and female halves, but using two different parts can create several inefficiencies and complications, such as
By standardizing single-use genderless connectors, end users can accomplish more with fewer finished goods, save time, reduce overhead costs, and get their biopharmaceutical products to market more efficiently. Benefits include
Overall, genderless connector standardization will help bioprocessing facilities simplify their operations and implement single-use technology to its full potential.
Todd Andrews
is the global sales and business development manager, Bioprocessing at CPC, 1001 Westgate Drive, St. Paul, MN 55114; 1-651645-0091;
[email protected]
.
Clarification of fermentation broths is one of the most important steps in bioprocessing. The first purification step after fermentation is the cell harvest, which is designed to remove cells and cell debris as well as to reach maximum product yield in compliance with existing regulatory environments. Standard technologies (centrifugation, separators, membrane, and depth filtration) can no longer handle the high particle loads (>10
8
cells/mL) in an economical way.
Deciding on the right purification system involves addressing questions about process performance, economics, and existing regulatory requirements. Process performance challenges include higher and higher cell titers, cell debris content, scalability, and flexibility for process changes and future processes, higher product yields, and constant high-quality product flow for further downstream purification.
Alluvial filtration (see box below) is a well-established and economical method in pharmaceutical industries (plasma fractionation). Use of ...
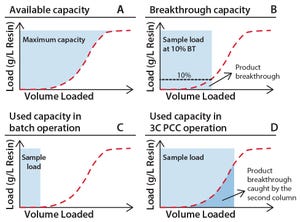
Interest in continuous bioprocessing is growing rapidly, and such an approach has shown to have a potential in providing gains in both productivity and savings in cost of goods sold. The ÄKTA™ pcc 75 chromatography system can be used for purification of target proteins in continuous downstream processes using periodic counter-current chromatography (PCC). PCC enables greater use of chromatography resin capacity, allowing sample loading to much higher levels than what is possible in traditional batch chromatography by using safety factors for loading (Figure 1). This study shows how the dynamic control functionality of ÄKTA pcc 75 can be used to adjust for variations in binding capacity of chromatography resin or for changes in feed composition when, for example, perfusion cell culture is being used. In this study, dynamic control was used for a protein A monoclonal antibody (MAb) capture step operated in a three-column PCC (3C PCC) setup.
Figure 1: Capacity use for standard and PCC; (A) total available ca...
In batch-mode chromatography, operating conditions do not allow full use of the total media capacity (
1
,
2
). Bio-Sequential Chromatography (BioSC®) is an open-loop process for the separation of multicomponent solutions (
3
). The unique column of the batch mode is replaced by a series of smaller columns (from two to six). This design maximizes the use of the media without product loss along the process sequences. The BioSC® Lab automated system is based on the multicolumn chromatography process, designed and provided by Novasep. Studies have been performed to demonstrate the benefits on IgG purity of a downstream process (DSP) monoclonal antibody (MAb) platform with two continuous chromatography steps (
4
). This note features the outlines of the method used and the qualitative and quantitative results obtained for the product critical quality attributes (CQAs) (Table 2).
Analytical Methods
Table 1: Batch and recipe targets generated by BioSC® Predict software
Feed Description:
IgG1 subtype MAb was p...
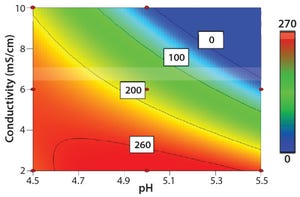
Cellulose is well known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. In addition, cellulose particles have unique pore-size characteristics appropriate for chromatography of biopharmaceuticals. Ion-exchange chromatography (IEX) is an important step for biopharmaceutical manufacturing. Specifically, cation-exchange chromatography (CEX) can be used as a capture step in monoclonal antibody (MAb) purification. Recently, advanced IEX resins have been developed using polymer modification techniques. Initial screening of conditions such as pH and ionic strength is important for IEX resin. The objective of this study is to reveal the differences of adsorption properties in polymer-modified, cellulose-based CEX resins.
Figure 1A: Contour plot analysis for optimal adsorption condition in Cellufine MAX GS resin
Screening for Optimal Adsorption Conditions with Cellufine CEX Resins
Cellufine MAX S-h (dextran modification) and Cellufine MAX GS (graft...
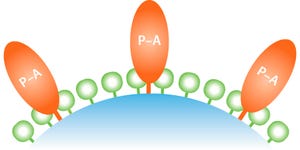
Currently more than 70 biosimilar monoclonal antibodies (MAbs) are under development, and multiple originator MAbs are going off patent in the next three to four years. Protein A affinity media material remains the most important workhorse for the purification of monoclonal antibodies.
Photo 1: Amsphere A3 is a new protein A resin designed with a surfacemodified base bead and alkali-resistant optimized ligand.
Protein A media has a high impact on both development and manufacturing costs, particularly during early stage clinical phases. This note summarizes key performance parameters for JSR Life Science’s Amsphere A3 high-capacity protein A resin for six biosimilar molecules of which five are MAbs, and one is a Fc-fusion protein.
Figure 1: Test conditions of the chromatography process
Material and Methods
Table 1 lists the MAbs and fusion protein used in this study, and Table 2 shows the experimental methods and materials. Figure 1 outlines the steps of the chromatography process and the test conditions. ...
Levitronix® has released the first ultrasonic, single-use (SU) flowmeter on the market. Characterized by high accuracy and superior performance, LEVIFLOW® SU noninvasive flowmeters enable a highly precise and low-cost flow measurement. With an accuracy of <1% of reading and flow ranges from 1 mL/min to 80 L/min, they offer the highest level of accuracy for measuring liquids in biopharmaceutical and other applications that require FDA, USP-VI, BSE/TSE, and animal-component–free materials that can be gamma sterilized.
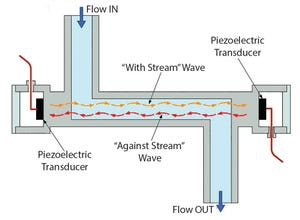
Figure 1: Operating principle of ultrasonic single-use sensor
Technical Background:
Figure 1 illustrates the operating principle: Two piezo-electric transducers, mounted in the sensor housing, generate and receive an ultrasonic wave. The wave going in direction of the flow (with-steam wave) is accelerated, and the wave going against the flow direction (against-steam wave) is slowed down. Both waves are processed by a signal converter. The difference of the transit time of both waves is propor...
The new LEWA EcoPrime® LPLC chromatography and enhanced buffer dilution platform combines proprietary fluid design and the LEWA Intellidrive® pump technology to deliver unrivaled reproducibility and accuracy. The integrated buffer in-line dilution (BID) option prepares point-of-use buffers combining two unit operations into a single, space-saving skid.
A single LEWA EcoPrime system has the flow range of up to two conventional LPLC units while delivering gradient accuracy of greater than ±1%. Because of its dynamic range, EcoPrime LPLC can bridge the gap between process development and pilot and production scales. The platform is highly configurable with 20 standard options to match your individual situation: full drainability, clean-in-place (CIP) enhancement, multiple inlet/outlet valve choices, precolumn analytics, DeltaV communication, and UPS ready are a few of the many user-configurable options. The EcoPrime LPLC series of systems provides significant flexibility, more floor space and — as a result o...
Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification.
Pall Life Science’s Cadence™ BioSMB PD platform is the first disposable flow path, continuous multicolumn chromatography solution that is fully scalable from a process development laboratory to good manufacturing practice (GMP) manufacturing. It provides an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps.
Figure 1: Principles of simulated-moving bed technology
During multicolumn countercurrent (also known as simulated moving bed, or SMB) chromatography, all chromatographic steps normally run in a batch mode are conducted simultaneously in different “zones” (Figure 1). Chromatographic media reaches full lifetime within a campaign through rapid cycling, making disposable use of even the most expensive chromatographic media possi...
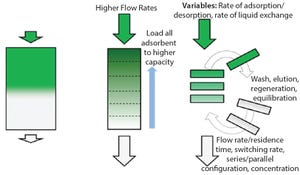
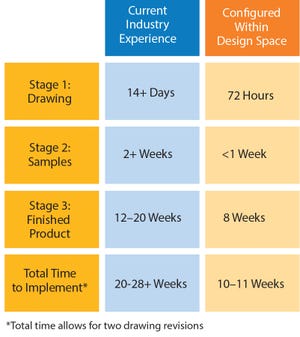
In June 2016, Parker domnick hunter began manufacturing in its new single-use facility in Birtley, Co Durham, UK. Here I discuss the approach taken in the design, testing, and manufacturing of single-use assemblies and some of the advantages they can have in terms of lead times, inventory control, and supply chain robustness.
Challenges of Customization
For some time, the message relayed to biopharmaceutical manufacturers was to design exactly what you want and need for each process. That meant a customized solution for every operation, for every scale, and for every product. There is no doubt that single-use technology has dramatically increased the flexibility of biopharmaceutical manufacturing facilities, but this has led to some challenges for the implementation of single-use systems.
High levels of customization can mean that organizations have to stock and control a large number of low-volume items, including managing and (where necessary) writing off expired stock. In addition, the requirement for ...
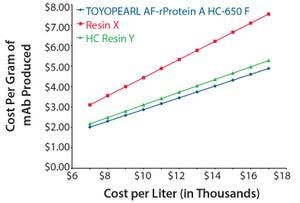
TOYOPEARL AF-rProtein A HC-650F and two other commercially available protein A resins (one of them also a “high capacity” resin) were compared on a cost-per-gram of monoclonal antibody (mAb) produced basis at multiple resin prices from $7,000 to $17,000 per liter as well as three different column configurations. For in-depth comparison, the median resin price of $12,000 per liter was used as a basis to determine comparative production costs among the three resins tested. The configuration used to model what the resin costs would be per gram purified was a column that was packed to have equal column dimensions of 36 cm ID × 15 cm.
Figure 1: Columns of equal dimensions
For this evaluation, the following values were held constant: residence time = 3 minutes; dynamic binding capacity (DBC) = 3 minute residence time (from product literature); column load = 80% of stated DBC; harvest titer = 3 g/L; harvest volume = 2,000 L; column lifetime = 100 cycles; column yield = 95%.
As shown in Figure 1, at a column capa...
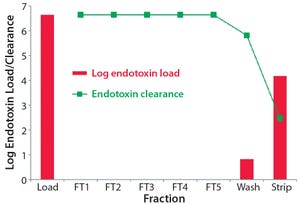
Various unit operations are needed in downstream processing (DSP) of biologics to remove process‑related impurities such as host‑cell proteins, nucleic acids, viruses, and endotoxins. This application note describes the potential of a salt‑tolerant anion‑exchange resin to achieve outstanding clearance of endotoxins and viruses.
Introduction
Endotoxins are lipopolysaccharides (LPS) originating from cell membranes of Gram‑negative bacteria. They are frequent contaminants of recombinant proteins produced in microbial expression systems. On the other hand, expression of more complex proteins in eukaryotic systems carries an inherent risk of introducing adventitious viruses.
TOYOPEARL NH2‑750F, a salt‑tolerant anion‑exchange resin, is ideal for intermediate purification of monoclonal antibodies (mAbs) and other proteins. Aggregates and negatively charged impurities, such as DNA, viruses, and endotoxins can be removed from a target of interest.
Figure 1: Log endotoxin clearance for each step and the correspondi...
Sponsored Content
Alcami specializes in all phases of pharmaceutical development — from critical preformulation studies to commercial product life-cycle management. Over the past 30 years, Alcami has supported more than 500 investigational new drug (IND) filings and over 50 new drug applications (NDA), abbreviated new drug applications (ANDA), and new animal drug applications (NADA). Our fully integrated, comprehensive development services are well established and ready to help our clients get the most out of their portfolios.
Principal Offerings
Drug Development
Alcami’s
analytical services
ensures that robust methods can be transferred throughout the world:
Alcami’s
formulation services
have proven know-how and timeliness for every development project:
Manufacturing and Packaging
API Manufacturing
Drug Product — Oral Solid Dose
Drug Products — Parenteral
Packaging Services
Support Services
Our in-house expertise in regulatory affairs and quality assurance can support your projects, inspections, and filings:
Catherine...
Sponsored Content
Associates of Cape Cod,® Inc. Contract Test Service (CTS) laboratory specializes in method development, bacterial endotoxin testing (BET), and glucan contamination. With the most extensive experience of any endotoxin testing laboratory in the world, CTS is GMP compliant, ISO registered, and licensed by the US Drug Enforcement Agency (DEA) as a laboratory capable of handling all controlled drug substances except those included in Schedule I. Endotoxin testing can be performed in accordance with US Food and Drug Administration (FDA), United States Pharmacopeia (USP), European Pharmacopoeia (EP), and/or Japanese Pharmacopoeia (JP), depending on your specifications.
In addition to routine testing, CTS has extensive expertise and the ability to customize endotoxin testing to individual client needs, develop methods for difficult samples and devices, transfer BET test methods, and design and produce custom depyrogenation controls for oven validations.
CTS has experience with the following
sample types
:
CTS qu...
Avid Bioservices is a contract development and manufacturing organization (CDMO) specializing in mammalian cell culture process development and CGMP production of clinical and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to the success of our clients, our team constantly strives to build partnerships that extend well beyond the delivery of your product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements
Established CDMO with Proven Capabilities
Thought Leadership in Process Sciences
Avid’s expanded process sciences group is focused on the ongoing development and enhancement of process and analytical methods needed to deliver optimized processes for transfer to CGMP manufacturing. Our experience with CHO-based antibody production and our knowledge of recombinant protein and enzyme process development provide the expertise you need to advance your programs to the next phase:
New State-of-the...
From drug and biologic development to delivery technologies and supply solutions, Catalent is the catalyst for your success. With 80+ years of experience, we have the deepest expertise, broadest offerings, and the most innovative technologies to help you get more molecules to market faster, enhance product performance, and provide superior, reliable manufacturing and improved results. Catalent is your strategic partner for biologic drug development and manufacturing success.
SMARTag™ technology
offers a new toolkit to optimize antibody–drug conjugates (ADCs) and bioconjugates. It allows site-specific, programmable drug placement using proprietary cytotoxin-linkers and conjugation chemistry in an efficient and scalable process. The result is improved ADC consistency, superior linker stability, and compatibility with any cell-based expression system.
Biomanufacturing Center of Excellence — Increasing Flexibility and Manufacturing Scale for Customized Solutions:
Our state-of-the-art biologics manufacturing...
byMin Park
Expertise
Cobra Biologics is a rapidly expanding international contract development and manufacturing organization (CDMO) of biologics and pharmaceuticals for clinical and commercial supply. With three GMP-approved facilities, each with expertise tailored to serving our customers around the world, we offer a broad range of integrated and stand-alone contract services stretching from cell line and process development through to fill and finish for the supply of investigational medicinal products and commercial production.
Experience
For over 18 years we have taken pride in being a trusted manufacturing provider, delivering what we promise and helping our customers to develop medicines for the benefit of patients. Cobra provides manufacturing technologies, platforms, and solutions for the pharmaceutical industry covering antibodies, recombinant proteins, viruses, phage, DNA as gene therapies, and whole-cell vaccines and therapeutics, as well a...
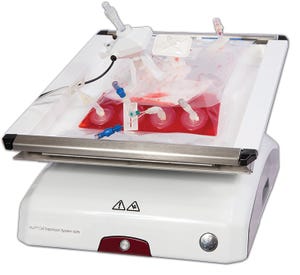
Ensuring optimal and maximal T-cell production is critical for adoptive immunotherapy and its continued success. The Xuri Cell Expansion System is an important component of the clinical manufacturing process. So we sought to investigate the effect of rocking rate and angle on the expansion of T cells. We used a combination of experimental data and predictive modeling and found that the rocking rate significantly influences the expansion potential of T cells with minimal contribution from the rocking angle. The results indicate that a rocking rate of 15 rocks per minute (rpm) and an angle of 6° are optimal for a 1-L bioreactor to maximize cell growth using a Xuri Cell Expansion System.
Introduction
The rapid and reproducible expansion of highly specific T cells from low precursor frequencies to clinically relevant numbers is essential for the success of autologous cell therapy techniques. The Xuri Cell Expansion System uses media perfusion and a rocking platform to achieve cell densities >10 × 10
6
/mL i...
bySam Kale
Biopharmaceutical companies face a broad range of challenges when developing new large-molecule products, including
Flexible Biomanufacturing
Patheon flexible biomanufacturing solutions offer a unique combination of expertise in development and manufacturing with advanced technological capabilities, innovative approaches, and flexible business models that can overcome challenges and reduce your risk.
Perfusion Processing:
Our expertise in mid-scale perfusion techniques allows for higher yields from low-titer cell lines as well as manufacturing of recombinant proteins with stability challenges, which result in higher product volumes at any given reactor size.
Single Use or Flexible Stainless Steel:
Single-use technology allows for faster, more efficient technology transfers; eliminates cross-contamination concerns; and simplifies management of your supplier relationship by streamlining your process. For processes that require stainless steel systems, our experienced staff achieves a 95% success rate to...
The pathway from conception to regulatory approval and commercialization of a cell therapy is long, complex, and resource intensive. To help inform numerous decisions along the way, an effective commercial manufacturing strategy for a cell therapy should be built on the four principles of what PCT calls development by design (DbD): quality, cost of goods (COGs), scalability, and sustainability. Proactively implementing a DbD strategy does not force a cell therapy developer to make a large manufacturing investment early in a project, but it does mean that the company must plan ahead. As a cell therapy moves through clinical development, working through the four facets of DbD at an early stage can provide significant cost and time advantages as well as effective management of comparability risk.
With DbD in mind, a prudent cell therapy developer will initiate and produce a strategic commercial manufacturing plan (SCMP) with the support of an experienced manufacturing partner. An SCMP provides a comprehensiv...
Rentschler is a full-service contract development and manufacturing organization (CDMO) and one of the leaders in the industry. Focused on the development and manufacturing of biopharmaceuticals in mammalian cell culture, we support our global clients through to market approval of their products. Delivering successful projects allows us to make an essential contribution to the global availability of biopharmaceuticals. Thanks to many years of experience, the high quality of our consulting services, and our flexibility, we can react quickly and effectively to clients’ requirements. Our success as a CDMO is underpinned by our focus on product quality, on-time delivery, and comprehensive and tailored project management. Founded in 1927, Rentschler is a privately owned medium-sized company headquartered in Laupheim, Germany.
InnovatIve Technology
Rentschler’s proprietary TurboCell™ mammalian expression platform enables the simultaneous production of up to 20 early stage drug candidate variants. The vector c...
Richter-Helm is a Hamburg, Germany–based contract development and manufacturing company with a proven 25-year track record, specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Our seasoned, 160-strong team supports you with process development, supply of products for clinical trials, commercial production, in-house quality control (QC) testing, and QP release. We operate two GMP-compliant production plants with bioreactor capacities of up to 1,500 L.
Richter-Helm consistently works to the highest standards of pharmaceutical quality, as verified by major regulatory bodies (European Medicines Agency (EMA), the US Food and Drug Administration (US FDA), Agência Nacional de Vigilância Sanitária (ANVISA), an...
Vetter is an international specialist in the production of aseptically prefilled syringe systems, cartridges, and vials. We are a family-owned, independent company and do not manufacture our own drugs.
Resources for Every Stage of Growth
Vetter’s full portfolio of services includes dedicated resources for both clinical development and commercial manufacturing. In addition, we provide expert packaging technologies and solutions tailored to your product’s specific market needs.
Vetter Development Service
Planning for Success:
Preclinical through phase 3 is a pivotal and unpredictable period for new molecules. Vetter Development Service helps smooth the path to clinical trials with dedicated support for key stages of development, testing, clinical manufacturing, and regulatory approval. We also help you integrate thoughtful, efficient life-cycle solutions for long-term growth and success. Vetter development service provides
Vetter Commercial Manufacturing
Filling Your Potential:
Both precise manufacturing ...
Wacker Biotech is “The Microbial CMO” — the partner of choice for contract manufacturing of therapeutic proteins using microbial hosts. Our service portfolio covers molecular biology, process and analytical development, and the GMP production of biologics for clinical trials and commercial supply. Founded in 1999 as a spin-off from the Hans-Knöll Institute in Jena, we are a 100% subsidiary of Wacker Chemie AG since 2005.
The sites in Jena and Halle provide a complete range of services for the development and GMP-compliant manufacture of biopharmaceuticals. Two manufacturing lines with 300-L and 1,500-L fermentation vessels and matching DSP scales are available to suit all possible customers’ needs.
WACKER holds biomanufacturing certificates from the relevant authorities for both sites and follows the ICH Q7A guidelines for GMP-compliant production of biologics. Since 2012, the facility in Halle has been EMA-approved/FDA-inspected for the commercial production of Reteplase (trade names Rapilysin® and Retav...