Sartorius 2014 Special Report
This special issue has come about through a long collaborative process with our contacts at Sartorius Stedim Biotech in Göttingen, Germany. It is the third such sponsored issue that BPI has published over the years with the company and the first one that we have produced in-house. We could not be happier with the results.
The aim here is to highlight technologies and responses to key industry challenges rather than focus over much on Sartorius itself. But the company’s long-standing culture of developing its own and integrating acquired technologies and processes — toward its goal of providing total biomanufacturing solutions — is worth underscoring. In the two interviews in this issue you will read about a company that has a long history of investing in and positioning its technologies and related equipment for future needs, even when the current industry has not been quite ready for them yet. In this risk-averse era, such long-term thinking can require no small amount of corporate courage. Through its o...
Automated Mini Bioreactor Technology for Microbial and Mammalian Cell Culture: Flexible Strategy to Optimize Early Process Development of Biologics and VaccinesAutomated Mini Bioreactor Technology for Microbial and Mammalian Cell Culture: Flexible Strategy to Optimize Early Process Development of Biologics and Vaccines
Photo 1:
ambr250 scale-down bioreactor system for parallel fermentation and cell culture
The use of mammalian and microbial cells in the production of biologics and vaccines is well established, and the majority of the top 10 drugs are now manufactured in this way. There is a significant and growing pipeline of new biologics (
1
), which in combination with increased pressure on cost reduction and generic competition from biosimilars (
2
), means that many biopharmaceutical companies are looking for ways to improve productivity in their development laboratories to ensure that upstream processes are efficient and robust.
One of the main challenges for those companies is to minimise the high cost of goods that is typically associated with cell culture production processes to ensure the commercial viability of a therapy. For example, monoclonal antibody (MAb) therapies are required in large doses (6–12 g) to achieve clinical efficacy. That means they can cost tens of thousands of dollars per patient per yea...
Photo 1:
UniVessel SU bioreactor with optical
holder and connection box
During the past decade, single-use bioreactors have become widely accepted as an alternative to conventional stainless steel or glass bioreactors for clinical manufacturing and process development. In the biopharmaceutical industry, glass bioreactors are used mainly for process development and optimization, but also scale-down models for process characterization. So it is of significant importance that such vessels replicate the design of production-scale bioreactors for both reusable and single-use applications. Stirred-tank bioreactors with 2-L, 5-L, and 10-L working volumes have proven to be particularly well adopted across the industry. The 2-L version is the work horse of process development, with a volume sufficiently large to serve as a representative small-scale model that allows sampling, yet is easy to handle.
Increasing Effectiveness of Development Staff
Project timelines and workloads can change dramatically, especially d...
Photo 1:
The ambr250 scale-down bioreactor system controls 12 or 24 disposable small-scale
bioreactors (100–250 mL working volume) and offers parallel processing and evaluation of multiple experiments while maintaining the characteristics of a larger scale bioreactor.
Design of experiments (DoE) is one of the most valuable techniques for organized and efficient planning, execution, and statistical evaluation of experiments. Although a DoE investigation can be completed using several runs in one bioreactor, small-scale bioreactor systems designed for parallel operation (such as the ambr15 or ambr250 systems) provide the optimal basis to economically realize a series of experiments. Because of the multitude of interdependent parameters involved in applications such as cell line development, culture media screening, and the optimization of bioreactor operating parameters, DoE evolved as an essential and indispensable method to create process knowledge. It has improved speed of development and has helped def...
During the past several years, single-use bioreactors have been gradually established in modern biopharmaceutical processes (
1, 2
). This adoption is directly linked to their unique ability to enhance flexibility and reduce investment and operational costs. Furthermore, production output can be increased, and time to market is shortened (
3
). Today a wide variety of single-use bioreactors exists for the cultivation of mammalian and insect cells (
4
), whereas only limited solutions are available for microbial cultures (
5
).
Figure 1:
ambr250, UniVessel SU, and BIOSTAT STR family; working volume ranges from 250 mL to 2000 L
Typically, processes are established and optimized in stirred-tank benchtop bioreactor systems. One challenge during the development of a robust cell culture process is the straightforward scale-up to final production scale. This is especially critical when using less-characterized bioreactor designs that deviate from the well-known and understood classical stirred-tank principle.
S...
Photo 1:
Different scales of single-use bioreactors with integrated, single-use pH and DO sensors; (left to right) UniVessel SU, BIOSTAT RM 20, and BIOSTAT STR 2000 systems from Sartorius Stedim Biotech
Through the past decade, single-use bioreactors for culturing mammalian and insect cells have been widely adopted in preclinical, clinical, and production-scale biopharmaceutical facilities (
1, 2
). With such bioreactors in operation, monitoring and control of process parameters is vital for ensuring critical quality attributes (CQAs) of biologicals or vaccines are met for production of a safe product. Traditionally, bag-based and bench-top vessels have been fitted with conventional pH and dissolved oxygen (DO) probes similar to those used in stainless steel or bench-top bioreactors. DO and pH are the most commonly monitored physicochemical parameters measured in real time. For many processes, pH measurement and control is critical because small deviations can influence culture growth and metabolism, par...
Figure 1:
Filtration principle of dynamic body-feed filtration (DBF) with diatomaceous earth (DE) (left) and conventional filtration (right)
In the past decade, biopharmaceutical manufacturers have demonstrated major improvements in monoclonal antibody (MAb) production, exhibiting product titers frequently in the range of 5–10 g/L using standard fed-batch mammalian cell cultures (
1, 2
). Increased product yields allow for smaller-scale production vessels. With 2,000-L single-use bioreactors already commercially available, single-use manufacturing of biomolecules becomes more and more an option. Other recent developments in the biopharmaceutical industry — e.g., drugs for smaller indications and more potent drugs allowing for lower dosages — will further stimulate the demand for smaller and more flexible single-use manufacturing facilities.
Although single-use technology in general has matured considerably over the past few years, some unit operations (e.g., cell removal) still need more attention to bec...
Left to right: Brian Caine (publisher of BioProcess International), Christel Fenge (vice president of marketing for fermentation technologies at Sartorius Stedim Biotech), and S. Anne Montgomery (editor in chief of
BioProcess International
).
Recently, BPI publisher Brian Caine and editor in chief Anne Montgomery had the opportunity to talk with Christel Fenge, Sartorius Stedim’s VP of marketing for fermentation technologies, in the Göttingen, Germany, Sartorius facility. They began by talking about fermentation technology, a topic that led them to touch upon a number of key issues in the biopharmaceutical industry.
Fermentation Technology
Caine:
Because this special issue focuses on fermentation technology, let’s begin by talking about some recent technological improvements and what impact these developments will have.
Fenge:
The major change or “breakthrough” of the past decade is the increasing use of single-use technologies, which started in the early 2000s with the implementation of the Wave biore...
Figure 1:
Polyethylene film development and manufacturing strategy
During the past decade, single-use bioprocessing bags and bioreactors have gained a significant foothold in the biopharmaceutical industry because they offer a number of advantages over traditional stainless steel equipment, especially for clinical production, multiproduct facilities, and emerging economies. At the same time, some companies are concerned that plastic materials might release potentially toxic substances that could affect cell growth and product titers (
1
). In a worst-case scenario, they could even compromise drug safety when a company uses disposable bags for intermediate or drug substance storage.
Despite the broad acceptance of single-use bags in clinical and commercial biomanufacturing, some researchers have reported recently on inconsistent and poor cell growth of some production cell lines in various single-use bags (
2–4
). Hammond et al. identified a degradation product derived from a commonly used antioxidant tha...
Figure 1:
Film and bag development based on a quality by design (QbD) approach, with expertise in material science, application know-how, film, weld, and bag making
With the increased use of disposable bioprocessing bags in all critical process steps of the biopharmaceutical drug production, there is a growing requirement for high-quality, robust, and easy-to-handle bioprocessing bags. The new generation of films and bags must combine multiple mechanical, physical, and chemical properties to make these products suitable and scalable for all processing steps in upstream, downstream, and final filling operations, including cell culture in rocking motion and/or stirred-tank, single-use bioreactors as well as storage, mixing, shipping, and freezing.
In partnership with our resin and film suppliers, Sartorius Stedim Biotech’s polymer and film experts have selected the best raw materials, optimized the design space of the extrusion process, and defined the critical welding parameters following the principles o...
Figure 1: Material science and quality by design (QbD) approach for development of Flexsafe bags
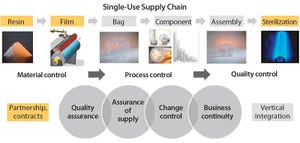
Growing adoption of single-use bags in commercial production of biopharmaceutical drugs raises new challenges for bag suppliers and drives the need for consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, polymer scientists and biologists at Sartorius Stedim Biotech have followed a stringent material science and quality by design (QbD) program to develop a completely new polyethylene film and to achieve consistent performance of new Flexsafe bags for all bioprocess steps and applications.
Reliability of the supply chain for Flexsafe bags is based on partnerships and agreements with suppliers and our complete understanding and control of the bag manufacturing process — from resin and film extrusion to final sterile bioprocessing bags. Consistent product performance is ensured by establishing sp...
Photo 1:
BIOSTAT STR 2000 bioreactor
During the development of single-use, stirred-tank bioreactors (e.g., BIOSTAT STR bioreactors), different phases can be distinguished (Figure 1). First, a clear definition of the intended application and all related requirements should be captured in a user requirement specification (URS). Based on that, the single-use bioreactor design phase and the material selection phase are initiated, both closely linked to each other. During the proof-of-concept phase, relevant component- and product-based tests are established and realized to ensure URS compliance. Finally, the qualification can be divided into product and process qualification, in which the latter includes qualification of the production equipment procedures and parameters. This process, together with stringent change-control procedures, will positively influence batch-to-batch consistency and security of supply.
Figure 1:
Phases during product
development of a single-use bioreactor
Final product qualification...
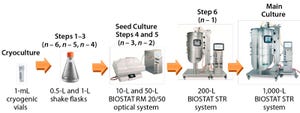
Figure 1:
Seed train of the 1,000-L fed-batch cell culture run starts with one cryogenic vial before six consecutive cell-expansion steps using single-use shaker flasks and bioreactors. A 17-day fedbatch production process followed in a BIOSTAT STR 1000 system. The entire duration from cryogenic vial to 1,000-L harvest on day 17 took 35 days.
In the past decade, single-use bioreactors have gained wide acceptance for biomanufacturing. The biopharmaceutical industry is increasingly interested in performing modern production processes in single-use facilities. That trend is driven by the time and cost benefits of single-use technologies, as well as the enhanced manufacturing flexibility they offer (
1
).
With single-use bioreactors increasingly used in late-phase clinical trials and commercial production, their quality, reliability, and assurance of supply becomes more critical. Many industry experts consider process control of film and bag manufacturing and traceability of raw materials to be key to ensuri...
Figure 1:
Porous fleece prevents masking effect
Single-use technology is well accepted today, and manufacturers’ quality assurance programs ensure leak-free single-use bags upon delivery. But what about risks involved with installation and other handling errors? Operator training and implementation of suitable standard operating procedures (SOPs) are mandatory, but should they be the only ways to mitigate the risk of failures? In addition, more companies are advocating the use of ballroom concepts (
1
) for the manufacture of biopharmaceutical drug substances and drug products. However, how do you prove that applied unit operations are closed and intermediates are not subjected to risks of cross-contamination?
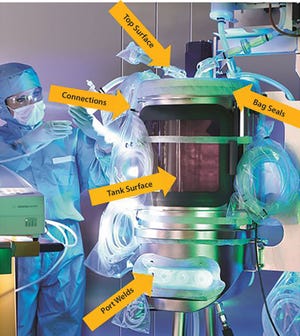
The high value of growth media and the duration of typical cell culture processes call for the highest degree of scrutiny when setting up such runs. A leaking bioreactor would generate a significant financial loss and jeopardize a carefully timed production schedule in a good manufacturing practice...
Left to right: Stefan Schlack (senior vice president of marketing and product management, Sartorius Stedim Biotech), Brian Caine (publisher of BioProcess International), S. Anne Montgomery (editor in chief of BioProcess International),
and Reinhardt Vogt (executive vice president of marketing, sales, and services, administrative board member of Sartorius Stedim Biotech).
While attending a conference at Sartorius Stedim Biotech in Göttingen, Germany, BPI publisher Brian Caine and editor in chief Anne Montgomery spoke with Reinhard Vogt (executive vice president of marketing sales and services, and member of the administrative board) and Stefan Schlack (senior vice president, marketing and product management). They discussed Sartorius’s forward-thinking business strategies, its position as a total solution provider, and how the company’s strategic goals mesh with its assessment of current industry directions.
Single-Use As an Enabling Technology
Caine:
Your company positions itself as a total solution prov...
SARTORIUS AG (WWW.SARTORIUS.COM)
The supply scenario for many biopharmaceutical drugs such as monoclonal antibodies (MAbs) is changing. With the implementation of personalized medicine resulting in drugs for specific, high-responder subsets of patients, market volume per drug will decrease. In addition, increasing fermentation titers of up to 10 g/L for MAbs are leading to smaller fermentation volumes necessary to accommodate individual biopharmaceutical market demands. That results in approaches such as flexible production in campaigns or decentralization of manufacturing using similar facilities with low risk of tech-transfer issues for regional markets.
In this regard, single-use technologies (disposables) play an important role in how biopharmaceutical development and production, particularly from mammalian cell culture, can nowadays be performed. Except for some large-scale unit operations such as centrifugation, chromatography skids, and UF/DF operations, all process steps can be performed in dispos...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.