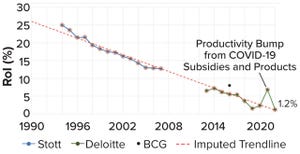
Voices of Biotech
Podcast: Sustainability at events is environmental and humanistic, says EBD
Life Sciences partnering events company EBD Group discusses the importance of communication, education, and the trials and tribulations of organizing an event with sustainability in mind.




.png?width=700&auto=webp&quality=80&disable=upscale)