July-August 2022
Founded in the middle of 2002, BPI published its first issue in January of 2003. Our 20th-anniversary issue thus takes the form of a state-of-the-early-21st-century industry report. Assembling this content has reinforced our appreciation of the remarkable paths that this industry has taken and continues to take — and our journey alongside it. Having worked as editors in this industry since August 1988 (Montgomery) and September 1996 (Scott), we’ve found it difficult to maintain a chronological grasp of developments — especially given how myriad transformations can arise from the uptake and incorporation of new technologies.
In the mid 1990s, the hot topic was validation — and for a time, biopharmaceutical publications were inundated with manuscripts on the topic as the industry grappled with the regulatory requirements. In this century, of course, the arguably disruptive topic has been single-use technology (SUT) — which now seems to permeate applications throughout drug development and manufacturing.
BPI...
Richard Johnson
The past 20 years have been a period of rapid change and development in the world and in the biopharmaceutical industry. One of the biggest changes has been the introduction of a pathway for biosimilars in the decade after 2010. Before that, no provision for “generic” versions of biologics existed. The European Medicines Agency (EMA) approved the first monoclonal antibody biosimilar in 2013, and the US Food and Drug Administration (FDA) followed with its first approval in 2015. Those approvals improved access for those therapies.
The development of single-use systems also has been critical in enabling rapid scale-up and scale-out of biopharmaceutical manufacturing. Stainless-steel reactors are notoriously expensive and have long lead times, so single-use technology has increased manufacturing capacity rapidly.
Improved characterization of large molecules also has enabled improved process understanding and increased yields. Use of modeling and big data has led to efficient product and proce...
Advanced analytics and the standardization of single-use systems have transformed bioprocessing
Biopharmaceutical analytical methods have improved significantly since the early 1990s. Techniques and fundamental mechanisms largely have remained the same, but modern analytical tools are delivering better insights into drug-product characteristics thanks to improved reagent quality, method intensification, and automation. Harmonized regulations and the revolutionized role of contract development and manufacturing organizations (CDMOs) also continue to support bioprocess transformations.
Improved Analytics and Standardized Single-Use Equipment
Today’s
analytical methods
provide high resolutions and deliver reliable results quickly. For example, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) used to be a laborious process that required practice to cast gels consistently and to quantify results using image analysis. But modern capillary electrophoresis (CE) systems are rapid, robust, and...
WWW.SARTORIUS.COM
As a trusted partner in the life-sciences industry, Sartorius has contributed significantly to the evolution of biopharmaceutical manufacturing. To reflect on the rich history of the industry, we arranged a virtual roundtable. Based on shared questions, we corresponded about what we considered to be the most important scientific, technological, and operational developments in the past 20 years of bioprocessing. We also reflected on the emergence of new modalities and how such products are likely to shape the future of the biopharmaceutical industry.
What Scientific and Technological Advances Have Been Most Formative to the Biopharmaceutical Industry’s Development?
Horry:
Many innovations have been implemented to increase upstream yields, improve product recovery, enhance product purity, and streamline manufacturing processes. In the early days of monoclonal antibody (MAb) production, for instance, protein yields from perfusion-mode cell-culture campaigns were around 100 mg/L.
Now, perfu...
Brian Mullan joined
Yposkesi
, an SK pharmteco company, as its chief technical officer in 2020. With over 20 years of experience, he has held leadership roles in late-phase process development, product launch, and commercial supply for large pharmaceutical companies making therapeutic monoclonal antibodies (MAbs). In eight years at Novartis, Mullan held posts as head of manufacturing science and technology and as a global technical project leader. From 2008 to 2012, he was process technology-transfer lead at Eli Lilly and Company. Before that, he worked at Centocor Biologics and Sanofi-Aventis. Mullan holds a 2001 PhD in viral genetics from University College Cork in Ireland and a 2011 MBA from the Open University Business School in Milton Keynes, UK. He conducted industrial postdoctoral work in adenoviral and adenoassociated virus gene-therapy vectors at Sanofi-Aventis. Below are his personal and professional reflections on the past 20 years in the biopharmaceutical industry.
Company and Industry Reflec...
Rentschler Biopharma headquarters in Laupheim, Germany
Rentschler Biopharma began its role as a contract development and manufacturing organization (CDMO) for biopharmaceuticals in 1997. Founded in 1872 as a pharmacy in Laupheim, Germany, the company transitioned to pharmaceutical manufacturing, then branched out into biotechnology, and ultimately channeled its expertise and experience into becoming a global CDMO. Our success over the past 150 years has been based on a strong foundation of creating value sustainably, and that has paved the way for further advancement and success. As a reliable partner and a passionate enabler, Rentschler Biopharma transforms innovative ideas into life-saving biopharmaceuticals. By connecting experts, experience, and expertise, we are mastering complex problems to achieve the best solutions.
Today, we are a globally leading, premium full-service company that understands the complexity of biopharmaceutical manufacturing. We leverage our long-standing expertise to create val...
SUTs such as Biosafe RAFT bags in
combination with Biosafe ports enhance sterility assurance in aseptic processing
A long-time editorial advisor for BPI, Miriam Monge is head of marketing for fluid-management technologies (FMT) at Sartorius, where she works to develop sustainable growth strategies for single-use bioprocessing. Over the past 20 years, she has played a significant role in championing development, promotion, analysis, and adoption of single-use technologies (SUTs). We asked her to share her experiences and to offer additional thoughts about current and future industry trends.
Science and Technology
From a technological perspective, without a doubt, I cite the implementation of SUTs in bioprocess development and manufacturing. Those technologies are gradually replacing stainless steel or glass pretty much throughout bioprocesses and are making an impact on the industry in so many ways. The industry had been stuck in a rut for such a long time operating massive stainless-steel facilities that ...
CPC’s portfolio of single-use connection solutions for biopharmaceutical applications
Single-use technology (SUT), specifically its evolution from clinical applications to commercial production, has made a remarkable impact on bioprocessing. SUT flexibility and sterility have helped to build a strong foundation for manufacturers to initiate production of many different drugs quickly and cost-effectively, including vaccines and cell and gene therapies (CGTs).
Single-use systems make it economically feasible to manufacture therapies for diseases that affect relatively small patient populations — the kinds of numbers that might not justify an investment in a traditional stainless-steel operation. SUT users also can produce multiple drugs in one facility, addressing a much broader range of therapies than would have been possible before SUTs were implemented.
During the COVID-19 pandemic, SUTs have provided biomanufacturers with a level of flexibility that will prove to be just as valuable and necessary during...
Purillex bottle microbial contamination testing
The choice of materials to develop and process biopharmaceutical products has a significant influence on the quality and purity of those products. Biomanufacturers have benefited from their use of both stainless-steel and single-use materials for individual process components and entire process systems. But careful attention must be paid to material characteristics. Working with different single-use plastics, for example, means that biomanufacturers must take product-contact issues into account, including the risk of extractables and leachables.
To celebrate its 20th anniversary,
BioProcess International
asked industry suppliers to participate in a questionnaire. Below, I provide my insights on the important technical developments defining the biomanufacturing industry over the past two decades and what other industries can learn from those developments.
What Material Science Development Has Changed Your Work?
Use of advanced materials in bioprocessing app...
Sponsored Content
Single-use bioprocess container protected in a robust shipping and storage (RoSS) shell
The evolution of single-use technologies (SUTs) is among the most important developments during the past 20 years of biomanufacturing. Large stainless-steel containers have long been used for storage and cooling of drug substances. However, there has been a shift toward SUT, which has enhanced manufacturing agility and flexibility. Establishment of single-use systems also has built a solid foundation upon which to improve production of several types of biopharmaceuticals, including mRNA, allogeneic cell therapies, and viral-vectored gene therapies. Especially for emerging modalities, single-use equipment is a logical choice. Manufacturers need to fill, freeze, and ship such therapies quickly. SUTs enable multimodal production, enhance throughput, and expedite changeover procedures. Disposable bioprocess systems also help companies to work flexibly, breaking up conventional batch sizes.
Single Use Support stands for cus...
It has been two decades since the theatrical release of the first Spider Man film with Tobey Maguire, the euro became the official currency for the European Union, and Anne Montgomery and Cheryl Scott began piecing together the inaugural issue of
BioProcess International
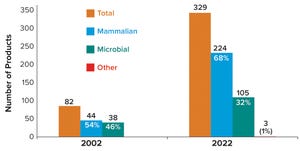
. In celebration of the 20th anniversary of this staple publication in the bioprocessing arena, we compared products and manufacturing capacity in 2002 with those in 2022 and delved into what has driven the changes that have occurred.
The biopharmaceutical industry, its state-of-the-art technologies, and the world at large all have changed since 2002. On the product side, the industry has witnessed an increase in the proportion of recombinant therapeutics sourced from mammalian cells, the emergence of antibody-based products expressed in microbial systems, and an explosion of mammalian-expressed antibody-based products. On the manufacturing capacity side, the industry has generated significant growth in mammalian manufacturing capacity, ...
Hardware and software are the focus here in Part 1.
HTTPS://STOCK.ADOBE.COM
The past couple of decades have witnessed significant advances in upstream bioprocess technologies and approaches. Since its establishment, BPI has been a facilitator of discussion in both print and professional conferences, as well as in webcasts and news online. To mark the 20th anniversary of the publication, we surveyed articles published over the past two decades and found hundreds that highlight significant advances in both emerging and established themes in biopharmaceutical production:
• “hardware” and assets (e.g., analytical instrumentation, bioreactors, and facilities) — in Part 1
• “software” and knowledge (e.g., process controls, quality by design (QbD), and process analytical technologies (PAT)) — in Part 1
• “wetware” technology (e.g., expression systems, cell-line development, culture media, and emerging modalities such as cell and gene therapies) — in Part 2.
Advances in single-use technologies (SUTs) are so exte...
Living cells are foundational technologies in the biopharmaceutical industry. They serve as hosts for production of therapeutic proteins, models by which to test clinical candidate efficacy and potency, tools for developing and validating bioanalytical methods, and even as medicines. To enrich our understanding of the past 20 years of bioprocessing, BPI distributed questions to supplier companies, including those that have spurred on innovation in the manufacture of critical starting materials. Below, representatives from ATCC reflect on advances in developing, banking, and authenticating cell lines and cellular by-products that are used in biopharmaceutical research, development, and manufacturing.
The Business of Biotechnology
Shalmica Jackson:
The global biopharmaceutical market is growing at an unprecedented rate and is predicted to reach a value of >US$400 billion by 2025, with a healthy compound annual growth rate (CAGR) of 8.1% (
1
). Most of that growth can be attributed to the rising prevalence ...
RHB employee
HTTPS://WWW.RICHTERHELM.EU
Richter-Helm BioLogics is a first-in-class biopharmaceutical CDMO with strong quality and customer focus. With 30 years of experience manufacturing microbially derived products — including product classes such as therapeutic proteins and peptides, antibody formats (e.g., VHH nanobodies), bacterial vaccines, and plasmid DNA (pDNA) — the company has gained first-hand knowledge that can be applied easily to individual customer projects. That positions it as a preferred and experienced partner, especially for plasmid DNA projects.
Over the past few decades, Richter-Helm has developed from a small biotechnology company into a leading contract development and manufacturing organization (CDMO) with a strong focus on customer needs, timelines, and quality requirements. It was one of the first CDMO players in pDNA production 30 years ago. Always on the cutting edge in a constantly developing market, the company implemented a number of production processes for protein- and p...
Jim Furey, general manager at PendoTECH
To celebrate the 20th anniversary of
BioProcess International
, industry suppliers were asked to respond to a questionnaire about the important technologies, trends, and manufacturing innovations that have shaaped their companies and the entire industry over the past two decades.
What has been the most important scientific or technological innovation in the past 20 years of bioprocessing?
I have been in the industry since the mid-1990s, and clearly the most significant evolution in biomanufacturing has been the widespread implementation of single-use technology. Many people would not have imagined making life-saving drugs and vaccines in 1,000-L plastic bags. The technology has moved far beyond plastic buffer- and media-storage bags and disposable filters. Single-use aseptic connectors, sensors, and other technologies have made single-use processing a reality. Such innovations cater to the needs of the industry to bring a plethora of biologically derived products ...
Equipment vendors, technology developers, and service providers have played an integral role in promoting innovation in the biopharmaceutical industry, from upstream production to final packaging and distribution of biological products. To enrich our understanding of the past 20 years of bioprocessing, BPI distributed questions to supplier companies. Below, Nick Pittman and Magnus Wetterhall of Waters Corporation reflect on advances in — and remaining opportunities for — process analytical technologies (PATs).
What Innovations Have Been Most Formative to the Past 20 Years of Biomanufacturing?
Wetterhall:
Although biopharmaceutical manufacturing involves multiple interdependent processes, improvements to cell lines and culture techniques have been and will continue to be the primary drivers of bioprocess innovation. Reliable, genetically stable, and high-producing mammalian and bacterial cell lines have improved upstream production significantly, as have high-quality serum-free culture media and new optio...
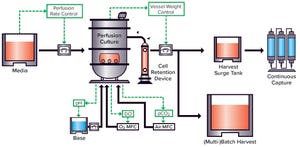
Recent global events have demonstrated a growing demand for biologics to be made rapidly, cost-effectively, and in high volumes. There has been an urgent need to develop highly flexible and cost-effective next-generation biomanufacturing solutions that provide high yields of therapeutic proteins. Novel technology platforms such as single-use bioreactors and continuous bioprocessing technologies have contributed greatly to improvements in product quality and productivity while reducing cost of goods.
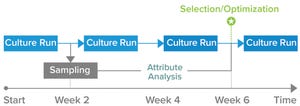
Continuous Bioprocessing Expands
Historically, continuous-culture processes were used for production of low-titer, low-stability, and labile proteins. Now the technology is applied increasingly to other biologics, including more stable modalities such as monoclonal antibodies (MAbs), bispecific antibodies (bsAbs), fusion proteins, and other recombinant proteins. Continuous biomanufacturing approaches have contributed to our ability to meet growing demands for biotherapeutics around the world. Perfusion cell cu...
Although single-use technologies have been part of downstream processing for at least 20 years, single-use centrifugation systems have been developed only in the past decade. They offer significant advantages over traditional centrifugation methods, and suppliers are developing single-use centrifuge lines for shear-sensitive applications. To enrich our understanding of the past 20 years of bioprocessing, BPI distributed questions to supplier companies. Below, Tiffany Rau (owner and principal consultant of Rau Consulting) provides her perspective on the breakthroughs in bioseparation and the advantages that can be gained from using a unique line of single-use centrifugation systems.
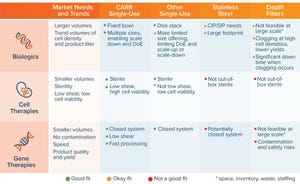
Figure 1:
Single-use versatility addressing multiple markets; important attributes of different technologies (CIP = clean in place, SIP = sterilize in place)
Breakthroughs in Bioseparation Systems
Until a decade ago, biomanufacturers had limited options for performing bioseparations. Technologies included bench-top, floor, fix...
HTTPS://WWW.SHUTTERSTOCK.COM
Over the past 20 years, the biopharmaceutical industry has made significant advancements in the way that biopharmaceuticals are produced. That principally has been driven by innovative new therapies and demands for greater availability of affordable biotherapeutics. Although the industry has made numerous performance improvements from cell line development and protein expression to bioseparations, downstream processing has presented the industry with its greatest obstacles.
For efficient processing and improved yields, step yields should be high (preferably >95%), and the number of unit operations should be limited, with no more than three chromatographic steps ideally. A purified product also must be of sufficient quality and purity to satisfy regulatory requirements. Given the amount of investment required to develop such a process, it is advantageous if it can be applied to different products that share common characteristics. That has given rise to platform bioprocesses, m...
Sponsored Content
Over the past 20 years, the bioprocessing landscape has undergone multiple transformations. Some of those were driven by biological innovations as new therapeutic platforms and modalities were introduced; others were driven by advancements in engineering and applied technologies such as single-use solutions, automation, and artificial intelligence. But the industry’s mission of making life-saving medicines that are effective, safe, and affordable remains the same. It’s rewarding to work in a field that aims to improve people’s health and life expectancies and provide a realistic hope of survival for those with terminal illnesses. Perhaps the most astonishing development and the best example of the industry’s capability is the development of COVID-19 vaccines. The speed at which the industry proposed innovative platforms to prevent or mitigate viral infection, moved projects swiftly through the development stages, tackled immense difficulties associated with regulations and governing agencies, scaled up pr...
Cygnus’s F550-1 Chinese hamster ovary host-cell protein enzyme-linked immunoassay kit
Different analytical methods have been developed to detect host-cell proteins (HCPs) in bioprocess streams. Advancements in such methods are enabling biomanufacturers to optimize their purification processes and ensure that their products are safe and efficacious. To celebrate the 20th anniversary of
BioProcess International
, Ken Hoffman (founder of Cygnus Technologies) provides insights into the history, trends, and future of HCP analytics.
History: 1990s to 2022
The recombinant therapeutic protein industry was in its infancy in the early 1990s. HCPs were recognized as bioprocess impurities that could cause adverse effects manifesting as immunological reactions or other off-target biological effects. Thus, it was known that HCPs should be measured and reduced to the lowest levels possible. Early US Food and Drug Administration (FDA) submissions for recombinant drugs in the 1990s used orthogonal methods such as two-dim...
The past 20 years have spurred new technologies that enable flexible solutions to changing market demands. Small-scale tools, improved analytical methods, and innovative facility designs are among the notable breakthroughs. To enrich our understanding of the past 20 years of bioprocessing, BPI distributed questions to supplier companies. Below, Elyse Vlahos (director of process engineering at Genesis AEC) provides her perspective on innovation and future industry developments.
Innovation and Regulations
What have been the most important technical innovations over the past 20 years in bioprocessing?
Facilities based on complex, large-scale stainless-steel bioreactors are evolving toward simpler sites with smaller-scale processes that can produce the same product volumes as traditional facilities within smaller footprints. Now with advanced therapy medicinal products (ATMPs) on the forefront, we see small-scale, patient-specific treatments with their own set of challenges. Such shifts have altered the traj...
Lindsay Smart, CEO of ZETA USA (right) and Andreas Marchler, CEO of ZETA Holding (left)
One of the most impactful innovations over the past 20 years of biopharmaceutical production has been the digital transformation of manufacturing processes. As executives of a solution provider for the pharmaceutical and biotechnology industries, we have witnessed the ongoing evolutions and applications of digital technologies. When digitalization is applied intelligently, time to market is shortened, and engineering, construction, commissioning, and qualification activities are accelerated.
Digital platforms
enable the aggregation of massive amounts of data derived from different sources such as engineering or process monitoring. Consistent, comprehensive, and detailed data acquisition and the provision of those data in digital form (e.g., as virtual images or a digital twin of a bioprocessing plant) can increase efficiency significantly. Biomanufacturers can address issues related to engineering, technology transfer...
Nick Green has worked in the global pharmaceutical and healthcare-services industries for more than 35 years, including significant experience with third-party manufacturing of biological products. He currently serves as president and chief executive officer (CEO) of Avid Bioservices, a contract development and manufacturing organization (CDMO) that focuses on biopharmaceuticals derived from mammalian cell cultures. He has held senior leadership positions at several life-science companies. That includes time spent as president and CEO of Therapure Biopharma, Evolve Biologics, and Rhodia Pharma Solutions; managing director of Nipa Laboratories; head of life sciences at Clariant International; and president of the pharmaceuticals division at Codexis. In his career, Green has supervised operations for more than
30 facilities spanning four continents. He holds a BSc in chemistry from Queen Mary University of London and an MBA from the University of Huddersfield, UK.
To enrich our understanding of how far the ...
Formerly known as the California Separation Science Society, the CASSS organization has maintained a close relationship with
BioProcess International
since its inaugural year. In particular, the CMC Strategy Forum series began the same year that BPI’s original staff joined Informa to develop a new international trade publication covering biopharmaceutical development and manufacturing. Beginning with our February 2004 issue, BPI has been proud to publish dozens of reports from this influential series of meetings over the years. Their success around the world — with similar events now held regularly in Asia, Europe, and Latin America — demonstrates the benefits of providing an open forum for scientific and technical dialogue that provides the basis for regulatory evolution.
Early in 2022, CASSS awarded distinguished fellowships to BPI editorial advisor Nadine M. Ritter (president and analytical advisor at Global Biotech Experts, LLC) and CMC Strategy Forum cofounder Mark A. Schenerman (president of CMC B...
BPI’s history coincides with that of biosimilars development. Although nonpeptide biosimilar products did not begin receiving commercial authorization until the 2010s, health authorities and drug makers already had been exploring the complex concept of biosimilarity. In the May issue of BPI’s first volume, Theresa L. Gerrard (then an independent consultant who also had been director of the Division of Cytokine Biology at the US Food and Drug Administration Center for Biologics Evaluation and Research, FDA CBER) wrote:
The potential for generic biotechnology products is both an exciting and scary prospect. Although some may argue that generic biotech products are not viable because of the inability to fully characterize them, others may argue that we are already at the threshold of such products because of current technology and policies on comparability. (
1
)
Gerrard described the FDA’s position on biopharmaceutical comparability and to anticipate scientific factors obstructing biosimilar development and...
Decades of research into cell biology, gene editing, and biomanufacturing have culminated in the commercialization of more than a score of cell and gene therapy (CGT) products. In the United States, most of those are hematopoietic progenitor cells (HPCs) isolated from human umbilical cord blood. As of July 2022, the US Food and Drug Administration has approved five products based on chimeric antigen receptor (CAR) T cells, all since 2017, and two viral-vector gene therapies, beginning with the 2019 authorization of Novartis’s Zolgensma (onasemnogene abeparvovec) (
1
). The FDA now is evaluating several candidates, including a few gene therapies that could receive approval by the end of 2022.
Despite landmark approvals, the advanced-therapy industry remains nascent. The novelty and complexity of such products create difficulties not only for their manufacture and quality control, but also for developing regulatory guidelines, governmental policies, and healthcare reimbursement models. To learn more about h...
For the past 20 years, I’ve been a committed and interested partner to developers of cell and gene therapies (CGTs). I’ve participated in the highs and lows of the industry. Initially, we looked to the development of monoclonal antibody (MAb) therapeutics for a roadmap to anticipate what would occur in the CGT field. It was thought that manufacturing processes would consolidate upon a single “winning” platform process and that both scale and productivity would be increased primarily by focusing on cell lines and culture media. Pioneers of the industry hoped that treatments would move from rare diseases and oncology to more encompassing indications. Ultimately, gene therapy developers found that different indications require different vectors because such delivery systems are designed for different functions. Likewise, we’ve moved from applying a single cell type such as T cells for chimeric antigen receptor (CAR)-based treatments to many different cell types, both native and genetically modified.
Clearly...
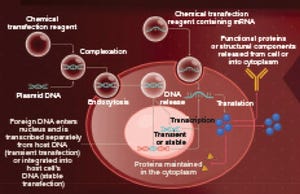
The science behind transfection spans from calcium phosphate precipitation to newer methods that are easier to perform, more efficient, and consistent. Mirus Bio strives to perfect gene delivery to cells in culture and support different applications within the life sciences community. The company’s capabilities include RNA interference (RNAi), clustered regularly interspaced short palindromic repeats (CRISPR), and viral vector development for cell and gene therapies with the launch of TransIT-VirusGEN GMP transfection reagent and kits for supporting clinical and commercial adenoassociated virus (AAV) and lentivirus (LV) production.
Founding scientists Jon Wolff, Vladimir Budker, and Jim Hagstrom spent most of their careers studying gene delivery to cells. They were instrumental in many landmark discoveries relating to in vitro and in vivo nucleic acid delivery and cell culture applications. In 1990, Wolff and colleagues at the University of Wisconsin were the first to show that naked plasmid DNA could be ...
Sponsored Content
To celebrate the 20th anniversary of BioProcess International, Tony Hitchcock (technical director at Charles River Laboratories) participated in a supplier survey on important bioprocess innovations, technologies, and advancements over the past two decades. He has over 38 years of experience in the biotechnology industry, specifically in production of critical starting materials and complex biologics for clinical trials.
What is the most important bioprocessing innovation in the past 20 years?
The emergence and adoption of single-use production systems has been important both for contract development and manufacturing organizations (CDMOs) and the sector overall. Although cross-contamination risk is a factor in the manufacturing of all biopharmaceutical products, that risk is especially significant for
Establishing reliable cleaning procedures for removing plasmid DNA (pDNA) and viral vectors is difficult because of the sensitivity of assays based on quantitative polymerase chain reaction (qPCR) to deter...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.