Happy autumn! I am writing this a few days before the editors head to Biotech Week Boston and the BPI conference. This is my first travel since January of 2020, and I am eager to see everyone again. As usual, the fall months coincide with our finalizing next year’s editorial calendar. I will be able to share those details (and other BPI news) with you in our next issue.
One major project that came our way a few months ago within our Informa Connect division was to incorporate into BPI a portion of the Institute for Validation Technology (IVT, formerly an Informa business) — and this process is still underway. Within the IVT Network,
The Journal of Validation Technology and The Journal of GxP Compliance
served the pharmaceutical industry proudly for nearly 30 years as a global knowledge base for validation and compliance professionals in FDA-regulated industries. The corporate decision, sadly, was to discontinue the network, its website, and both journals.
We are excited, however, to be adding a number o...
BPI’s first cell-therapy supplement was published in March 2011 (
1
).
WWW.BIOPROCESSINTL.COM
In the first cell therapy special issue of BioProcess International back in 2011, members of the International Society for Cell and Gene Therapy’s (ISCT’s) commercialization committee highlighted the need for cell-processing professionals who prepare bone-marrow and cord-blood products to collaborate with bioprocess engineers in establishing commercially-relevant manufacturing processes for a new wave of cell-based therapies (
1
). The emerging field of cell and gene therapy presented unique challenges for creating scalable bioprocesses under current good manufacturing practices (CGMPs) to accommodate primary cells with limited expansion potential, many of which were adhesion dependent.
The article highlighted a developing discipline,
cell therapy bioprocessing
. Publication of BPI’s special issue coincided with a flurry of activity within academic translational institutes and many small and large biopharmaceuti...
HTTPS://WWW.ALAMY.COM
The past 20 years have been extraordinary for intellectual property law. Most of the transformations can be attributed to changes in the statute, US Supreme Court jurisprudence, almost complete turnover in judges in the Court of Appeals for the Federal Circuit (“Federal Circuit,” below), and advances in technology that could not have been anticipated in 2002.
An understanding of those changes requires a retrospective of patent law over those 20 years. At that time, the Federal Circuit was 20 years old and had achieved what many believed was Congress’s purpose in instituting the court: patent law harmonization. While on the court, former Chief Judge Randall R. Rader wanted to enable business professionals to assess (with a patent attorney) the scope of a competitor’s patent to achieve a reasonable understanding of the risks that the patent posed to their commercial intentions. Claim construction had been determined to be a matter for the US Supreme Court, with de novo review by the Fe...
Cleaning validation receives a great deal of attention within the biopharmaceutical industry, not least because of the risks of product adulteration and hence patient harm from improperly cleaned surfaces (notwithstanding additional concerns such as operator protection). Traditionally, cleaning validation efforts focus on direct product-contact surfaces. However, the hazard and resultant risk posed from indirect product-contact surfaces should not be underestimated.
Consideration of the risks presented by indirect surfaces should figure into every contamination-control strategy. Understanding of these risks cannot be generalized easily because they depend on equipment design, can be influenced by operational modes (such as a vibrational waves), and even change as equipment ages. Industry surveys suggest that indirect product-contact surfaces are an underexamined area within cleaning validation (
1
).
Principles
To determine and demonstrate the degree of confidence that a given residue is removed cleaning ...
Sterilizing-grade filtration is an essential operation for biomanufacturing. It ensures that drug substances are free from microorganisms at the end of a downstream process. The COVID-19 pandemic has highlighted the need for high-quality therapies to be manufactured efficiently at scale, with particular focus on the need for multiple vaccines to be developed, produced, and distributed globally (
1
). Some vaccines have used lipid nanoparticle encapsulation technology, which also has potential for use in gene therapy development in the near future. Lipid delivery vehicles offer several therapeutic advantages that have contributed to the success of vaccine manufacturing worldwide.
However, lipid nanoparticles can present some difficulties during bioprocessing at the sterilizing-grade filtration step (
2, 3
). Lipid nanoparticles such as liposomes often are much larger than conventional biologics such as monoclonal antibodies (MAbs). That can be problematic when a particle’s size is reasonably close to the n...
Antibody–drug conjugates (ADCs) represent a growing therapeutic segment of the oncology field. Five such treatments received market approval from the US Food and Drug Administration (FDA) between 2008 and 2018, whereas three were approved in 2019 and two each were approved in 2020 and 2021 (
1
).
This disruptive technology combines highly potent small-molecule payloads with monoclonal antibodies (MAbs) to improve their specificity as cancer treatment. The antibodies deliver those toxic compounds directly to cancer cells but not to healthy cells, thereby mitigating many of the adverse effects associated with traditional chemotherapy. Once a MAb has bound to its target epitope on a tumor cell, it is internalized to merge with a lysosome inside the cell. Degradation of the resulting ADC–receptor complex releases the cytotoxin into the cell and causes apoptosis through a number of pathways (e.g., microtubule disruption and DNA damage).
Coupling payloads to MAbs requires fully purified antibodies, which are gr...
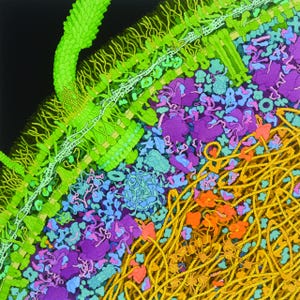
Depicted here as a lattice of proteins between the inner and outer membranes of an
Escherichia coli
cell, peptidoglycans and flagellar filaments are known to generate significant immune responses to biological drug products.
ADAPTED FROM IMAGE BY DAVID GOODSELL,
HTTPS://COMMONS.WIKIMEDIA.ORG/WIKI/ FILE:ESCHERICHIA-COLI-BACTERIUM(1).TIF
Process-related impurities such as host cell proteins (HCPs) can raise concerns about biological product efficacy, quality, safety depending on their properties and levels. In the first part of this series, we surveyed relevant regulatory frameworks and detailed potential effects of HCPs on biologic efficacy. Here in part 2, we review available literature on HCPs and patient safety, including information about HCP-related immune responses and adverse clinical events.
HCP Effects on Patient Safety
At least five HCP-induced factors can influence a therapeutic protein’s
safety profile:
• immunogenicity — activation of innate or induction of adaptive immunological reactions...
Microbiology has risen as a major part of global industry over the past three decades. Industrial microbiology, biotechnology, biopharma and now biointelligent production systems (
1
) embrace a wide range of manufacturing platforms and product areas involving microbes, animal cells, and plant cells — as well as whole organisms. The multibillion-dollar applications of biomanufacturing display great variety. They include microbial-based production of such valuable metabolites as amino acids, vitamins, solvents, and organic acids as well as larger products such as enzymes, vaccines, and antibiotics. Even larger protein molecules requiring eukaryotic secondary processing are expressed by higher animal cells, mainly Chinese hamster ovary (CHO) cell lines.
Recombinant DNA technology has improved many parameters in production of those products (
2
). Advanced molecular manipulations have been added to mutational techniques as a means of increasing expression titers and yields of both microbial and animal-cell–b...
Production of custom-design single-use assemblies for fluid management.
Most biopharmaceuticals are manufactured in large-scale stainless-steel piping and vessels, with downstream processes taking place within rigid and inflexible facilities. Although process steps such as harvest, purification, fermentation, filtration, dispensing, and freezing require flexibility, stainless steel has not been replaced yet by single-use systems at a large scale. However, manufacturers wanting to optimize process efficiency and scalability to obtain a viable and valuable product for commercial use inevitably will need single-use technology. Aseptic fluid management with single-use systems offers numerous immediate benefits and enables automated workflows. Such systems enable biomanufacturers to move toward advanced manufacturing.
Full Flexibility with Single-Use Systems
Implementing single-use systems in downstream processing enables flexible scale-up and facilitates process set-up. They are applicable to different manufa...
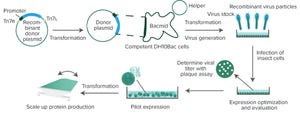
Proteins create cellular matrices, catalyze biochemical reactions, and form signaling pathways to respond to external stimuli. Studies of protein structure and function increase researchers’ understanding about the foundations of life. However, because most proteins are difficult to obtain commercially, it is important to establish sources that can provide researchers with plentiful supplies of proteins. In recombinant protein expression, a gene encoding a protein of interest is cloned into an expression vector (usually a plasmid) and transferred into a host cell for protein production by harnessing the cell’s intrinsic protein synthesis machinery. Several host-cell systems have been established for recombinant protein production. Selection of the optimal host for a given protein is a major factor for successful expression. Table 1 lists the advantages and limitations of commonly used expression hosts.
Table 1:
Commonly used host cells for recombinant protein expression.
Baculovirus Expression Vector Syst...
WWW.SARTORIUS.COM
It is no secret that progress toward intensifying monoclonal antibody (MAb) production processes has focused on upstream steps. Although the industry welcomed increased production, that also created bottlenecks in downstream processing, including during capture chromatography steps. Technologies that are intended to alleviate such bottlenecks must meet four important criteria to increase productivity and profitability. They must
• improve productivity of the MAb capture process, such as by purifying more MAbs, using less media, and/or reducing timelines.
• perform as well as or better than current technologies.
• prove to be suitable as a platform approach and performs consistently and reliably.
• scale easily; innovations in MAb capture should work in process development and large-scale manufacturing.
If a new method meets those criteria, then it offers a huge potential for increased production capacity and profitability in a MAb production process.
A 2022 publication in the journal
Me...
WWW.ISTOCKPHOTO.COM
The expectation to apply quality by design (QbD) principles to new manufacturing processes has been voiced by regulatory authorities for over a decade (
1, 2
). They recognize that because of the generally low patient populations for emerging therapies, such as adeno-associated virus (AAV)-based therapeutics, available chemistry, manufacturing, and controls (CMC) information might not be as exhaustive as for other biologicals such as monoclonal antibodies (
3, 4
). Other challenges include the need for rapid development to address currently unmet medical needs and the availability of suitable raw materials.
Frameworks regarding CMC requirements for gene therapy applications have been published by the Alliance for Regenerative Medicine (ARM) and the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL) (
5
). Pall also has produced a white paper on this subject (
6
). In that publication, the focus is on manufacturing AAV, outlining key steps for successful deve...
Extending the use of approved advanced-therapy medicinal products (ATMPs) to the tens of thousands of patients who could benefit from such treatment requires a 10- to 100-fold production scale-up. Given that each autologous ATMP batch yields one dose for one patient, expanding production throughput is not a question of boosting volume, but
rather of amplifying single manufacturing runs. That is, scale-up is actually scale-out, and the dimensions of the ensuing endeavor extend beyond what occurs in the cleanroom.
Coupled with each production run is a battery of controls for raw materials, in-process conditions, and product release. Higher throughput requires a smart facility layout that ensures segregated and controlled flows of materials, people, waste, and products. Increasing the number of processed batches by several times requires stringent process monitoring and validation based on expanded data pipelines.
In BPI’s September 2022 issue, part 1 of this report described a plug-and-produce (PnP) cell-m...
Photo 1:
Bench-top Octave BIO MCC instrument with SkillPak columns.
Biomanufacturers typically have relied on multistep processes for optimal removal of impurities such as host-cell proteins (HCPs), DNA, adventitious viruses, and aggregates. However, additional purification steps increase downstream expenses significantly, including costs of supplementary resin, hardware, and buffers. The substantial footprint required at a processing site and additional time needed to perform a complete multistep purification process also increase production costs and complicate process execution. Thus, it is imperative to design and test effective purification procedures for high-quality biotherapeutics, but reasonable process costs, time, and manufacturing space are required.
Batch purification platforms for monoclonal antibodies (MAbs) typically contain an affinity capture step based on protein A followed by polishing steps for further impurities reduction. Innovations in downstream MAb processing, including multicol...
On paper, scaling a bioprocess from a 10-L to a 100-L to a 2,000-L bioreactor may seem like a straightforward math problem that could be solved by software. In practice, however, the exercise relies on a complex set of biological, chemical, and engineering assumptions; on maintenance of healthy cell cultures; and on management of equipment and analytics while adjusting to each increase in scale (
1
). Process development and quality control groups need to monitor how scale-up might affect critical quality attributes (CQAs) of a drug substance (DS) — including host cell proteins (HCPs). The HCP profile can change during process scale-up, necessitating further adjustments to the downstream purification process.
Although many HCPs are benign, some are immunogenic. Others can interact with a DS and reduce effective product dosage, which ultimately poses some risk to patients and can limit drug efficacy and stability. Managing HCPs thus constitutes a significant component of each biopharmaceutical drug develop...
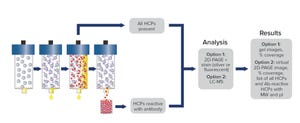
A well-developed, broadly reactive, and qualified enzyme-linked immunosorbent assay (ELISA) is the gold-standard method for ensuring removal of host cell proteins (HCPs) and demonstrating process consistency and final drug substance (DS) purity. Regulatory guidelines require sponsors to use orthogonal methods for demonstrating antibody coverage of individual HCPs and provide a comprehensive assay qualification package to ensure that the ELISA is fit for purpose. In a June 2022 webinar, Eric Bishop (head of R&D and custom development services at Cygnus Technologies) discussed best practices in HCP immunoassay qualification and bridging necessitated by critical reagent changes.
Bishop’s Presentation
After many years of performing 2D Western blotting for coverage analysis, Cygnus Technologies concluded that the method is not predictive of how antibodies would perform in an ELISA. So the company launched Antibody Affinity Extraction (AAE) technology in 2014 — and since then has incorporated it with mass spect...
As an entrepreneur, I stepped away from a fulfilling position in a large and established company to go out on my own and build something new. My decision to leave the advanced research department at
Illumina, Inc.
to create a start-up might seem like an extreme leap, but I am very familiar with the risks of starting over.
After earning my bachelor of science degree in biotechnology from the University of Tehran, I immigrated to the United States. I left my family and the only country I had ever known because I had a passion to pursue scientific breakthroughs that I could not make at home. Here are some key lessons I’ve learned along the way. I hope to inspire the next generation of innovators wherever they are in the world.
Entrepreneurial Lessons Learned
Speak up even before you “earn” the right to do so. Ask questions as they occur to you.
I first felt the pull of entrepreneurship when I was 10 years old. My father and uncle had started the first modern coffee shop in Tehran, serving lattes and cappu...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.