September 2021
WWW.LIFETOUCH.COM
Amid the continuing frustrations of this time, we are beginning to see what conducting business in a post-COVID-crisis world will be like. A new survey conducted by our Informa Connect Life Sciences group compares responses with those from similar questions in April 2020 — knowing more than we did before about the impact of COVID-19 around the world. The data reveal insights into how the global life-science community has responded to the crisis, the impact the pandemic has had on different parts of the biopharmaceutical industry, and how operations could be transformed for the better in the future.
Here are a few take-away insights from the new survey, but I encourage you to view the complete reports online (
COVID-19 survey 2020
|
COVID-19 survey 2021
).
Nearly half of the organizations represented are working on diagnostics, vaccines, and/or treatments for COVID-19. More Europeans and Asians responded this year than in 2020. About half of respondents note that vaccine approvals, prod...
Audits are a vital quality-management tool of the biopharmaceutical industry. Whether the objective is to verify supplier or partner qualifications, contribute to corrective and preventative actions (CAPA), or fulfill regulatory compliance requirements, conducting proactive auditing is key to successful operations.
Over the past year, virtual audits —also known as remote or distance audits — have enabled biopharmaceutical companies to meet compliance and quality assurance demands despite COVID-19–related travel restrictions and social-distancing protocols. Notwithstanding the challenges of virtual audits, the benefits that they provide are likely to make them a long-term practice.
Developing an effective strategy can help biopharmaceutical companies successfully navigate virtual audits. Such a strategy should begin with a risk-based evaluation of facility or supplier quality and remote-audit readiness. Next, a company should develop its complete and comprehensive plan for updating corporate procedures to ...
Manual processes rely on numerous manipulations using biosafety cabinets.
Cell and gene therapies (CGTs) offer potential cures to some of the most challenging illnesses of our time. The number of such therapies approved for market is set to surge in the next 10 years (
1
). Yet current manufacturing approaches are not fit for purpose. Biomanufacturing must adapt to prevent the industry from unintentionally sleepwalking into causing harm to patients. Some urgently needed changes could come with learning about the mindset of the medical-devices industry.
Background and Current State
After a strong start a decade ago, the number of approved CGTs on the global market today is fewer than had been expected. The challenge of designing adequate clinical trials and manufacturing processes for living medicines has proved to be a daunting one. Meanwhile, ethical and practical considerations related to access, value, and cost also have caused delays.
Biomanufacturing processes for cell and gene therapies have evolved...
Microbiome-based therapeutics have evolved significantly in recent years, with several promising candidates advancing through the clinical pipeline. That progress is the result of growing evidence showing that targeting and manipulating the microbiome could improve human health and treat more than 25 conditions by restoring healthy bacterial populations (
1
).
The human microbiome comprises a diverse community of microorganisms, helpful and harmful. It differs by person based on factors such as genetics, environmental influences, diet, and immune function. Microorganisms play a role in just about every biologic system, helping to digest food, produce certain vitamins, regulate the immune system, and provide resistance against infections. But pathogens, antibiotics, unhealthy diets, inflammation, and other factors can disrupt a person’s microbiome (
dysbiosis
) and trigger disease. Complex interactions among the human microbiome, health, and disease are being discovered continually (
2
,
3
).
Clinical res...
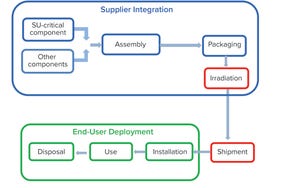
Figure 1: Product life cycle of single-use systems
Single-use (SU) technology plays an important role in modern vaccine and biologics manufacturing. System integrity, managed by critical process controls, ensures sterility and is a prerequisite for successful leak-free processing. Nonintegral systems cause loss of product, quality, and time; increase costs through investigations; and lead to potential safety problems. The BioProcess Systems Alliance (BPSA) issued a white paper in 2017, Design, Control, and Monitoring of Single-Use Systems for Integrity Assurance (
1
), that describes in detail the strategies for design and quality assurance with regard to system integrity within the single-use systems (SUS) life cycle (Figure 1). Several other publications address design and quality assurance for SUS from the broader perspective of system integrity (
2
–
4
).
The following four case studies focus on system-integrity challenges. The first example describes development of an improved pressure-decay test met...
Manufacture of biopharmaceuticals using mammalian cells inherently incurs a risk of viral contamination during cell cultivation. If introduced, viruses can infect and replicate in cells used to produce a therapeutic protein or vaccine. The consequences of such contaminations can be dramatic. Not only can a company lose contaminated batches, but it also faces potentially extensive root-cause investigations, facility cleanup efforts, and introduction of preventive measures. Until contamination issues are resolved adequately, production should not be resumed, and facility downtime brings significant economic and capacity implications.
Unintended introduction of virus into mammalian cultivation processes must be prevented at almost any cost. To achieve that goal, it is important to understand the entire biomanufacturing chain. Only through scrutiny of each aspect can a company identify possible risks for introduction and then effectively mitigate those risks.
The need to ensure viral safety during production ...
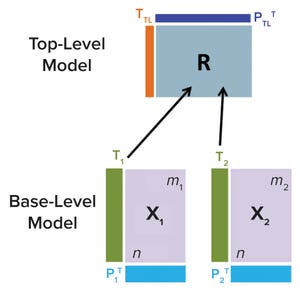
Figure 1: Hierarchical model schematic; information from all base-level models is communicated to a top-level model through their respective matrices (
T
j
) into a consensus matrix
R
.
R
is used to further summarize data from all base-level models by generating top-level scores
T
TL
and loadings
P
TL
.
Purification is an essential process in biopharmaceutical manufacturing that separates a therapeutic protein in its active form from impurities. A typical purification process consists of several chromatography unit operations, and each unit operation comprises multiple phases. During the operation of each step, continuous (time-series data per parameter for each batch) and batch data (one data point per parameter for each batch) are generated by in-line sensors installed in chromatography skids on the production floor and with at-line/off-line in-process samples, respectively. Such process data can be leveraged for developing advanced data-driven models that can provide insights for process experts ...
Bioprocessing 4.0, the biopharmaceutical version of Industry 4.0, is on course to become a reality in the next decade (
1
). This is because its “cyber-physical systems” that comprise cloud computing, connected systems, and digital process control offer many benefits. They include better process monitoring and management of a biologic’s critical quality attributes (CQAs) and the chance to control intensified processes for faster, less-expensive production of protein-based biologics and vaccines. Automation also can reduce the number of skilled operators needed while making manual tasks less error-prone and more efficient. That frees scientists to work on multiple processes simultaneously and respond rapidly to process issues, even from remote locations.
Bioprocessing 4.0 Roadblocks
For many biopharmaceutical companies, Bioprocessing 4.0 remains a bête noire that is insurmountable. Their perception is that the cost, resources, and time required to adopt best practices are just too much to justify without a...
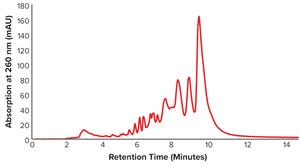
Figure 1: Anion-exchange high-performance liquid chromatography (AEX-HPLC) chromatogram of unpurified oligonucleotide sample with 42.17% purity
Oligonucleotide-based therapeutics have been studied over recent decades, and their promise as a new drug modality is now being realized. The growing interest in oligonucleotides is driven by their high potential for treating different medical conditions, the growing number of oligonucleotide drugs approved by the US Food and Drug Administration (FDA), an increased focus on personalized medicines, the development of therapies for rare diseases, and the wide adoption of nucleotide-based COVID-19 vaccines.
Oligonucleotides are short, linear sequences of DNA or RNA that generally are manufactured by chemical synthesis. Oligonucleotides are extremely susceptible to oxidation, enzymatic degradation, and clearance in vivo. Because of that, synthetic oligonucleotides often are chemically modified to improve their stability and make the molecules resistant to extracellula...
When considering strategies for expanding the number of cells being grown to support cell therapy development, companies often focus on decisions regarding
scale-up
and
scale-out
: increasing capacity either by using larger vessels to increase production volume or by implementing more units of the same vessel, respectively. Complete workflows often involve both. Figure 1 shows an example of scaling out from one to multiple cell culture flasks of the same dimension before transitioning to a larger format.
Figure 1: Scale-out of flask-based cell culture operations adds more of the same type of flask into a production workflow, whereas scale-up involves transitioning to a larger culture-vessel format such as a Corning HYPERFlask, CellSTACK, HYPERStack, CellCUBE, or Ascent FBR system or to spinner and shaker flasks depending on cell type.
Scale-out can be straightforward because the production unit remains the same; scaling up can be more complex and require more planning. Considerations include the time n...
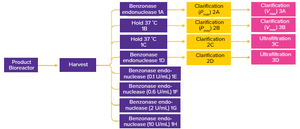
During production of vaccines and viral vectors, the size and quantity of extracellular nucleic acids must be reduced using endonuclease enzymes. Merck/MilliporeSigma’s proprietary Benzonase endonuclease is a genetically engineered nuclease derived from the Gram-negative bacteria
Serratia marcescens.
It attacks and degrades all forms of DNA and RNA. It is manufactured under good manufacturing practice (GMP) conditions and has a drug master file (MDF) in place with the US Food and Drug Administration (FDA), which can be cited in regulatory filings. In this supplier-side article, authors from Merck/MilliporeSigma summarize the benefits of incorporating Benzonase endonuclease in a Sabin poliovirus type 3 production process.
Specifically, the authors examine the amount of Benzonase endonuclease required to degrade Vero cell DNA and describe its effect on clarification-filter capacity. Their study also explores the endonuclease’s effects on Vero-cell DNA levels following tangential-flow filtration (TFF), the...
Swissfillon AG is a contract manufacturing organization (CMO) based in Switzerland. Fully compliant with current good manufacturing practice (CGMP) regulations, it provides state-of-the-art aseptic filling for pharmaceutical and biotechnology companies, from clinical-phase materials to commercial quantities. This CMO specializes in high-value, difficult-to-fill products.
Swissfillon recognized that adoption of single-use systems (SUS) on a commercial scale required major improvements in consistency and reliability compared to manual operations at pilot and clinical-trial scale. The single-use formulation and filling process, which includes an Optima multiuse filling system, was designed to be fully automated and highly flexible. It was finished in 2018, and a further expansion with a second filling line is scheduled to be installed in 2023–2024. The company can fill batch sizes between 1 L and 100 L now, and in the future will handle up to 500 L in vials, syringes, or cartridges.
The multistage system not...
Suitable scale-down perfusion systems generally have been unavailable for process development (PD) activities. Some commercially available systems require daily media exchanges. No such system performs in a way that accurately represents large-scale perfusion, and none maintains sufficiently high cell densities. Kevin Lee (cofounder of Erbi Biosystems) joined BPI on 4 May 2021 to explain how his company’s Breez bioreactor system integrates all the functions of a stirred-tank reactor (STR) into a compact format that can facilitate PD, enabling one person to run three to four times as many experiments as with an STR.
Lee’s Presentation
With a 2-mL working volume, the Breez bioreactor is one of only a few milliliter-scale perfusion systems. It consists of a single-use cassette bearing rigid microfluidic channels covered by a silicone membrane. Requisite bottles, pumps, mixers, tubes, filters, and sensors are integrated into the cassette such that the bioreactor is packaged as a presterilized, precalibrated “...
The number of cell therapy product candidates based on mesenchymal stem cells (MSCs) has grown steadily since their clinical debut in 1995. As of June 2020, clinical investigators were evaluating more than 1,100 such therapies. Scaling up MSC production remains challenging, however. On 31 May 2021, Hilary Sherman (senior scientist at Corning Life Sciences) presented an “Ask the Expert” webinar describing her company’s efforts to facilitate MSC workflows.
Sherman’s Presentation
Easing Expansion:
MSCs have strong differentiation capability and can be isolated easily from several parts of the body — often from bone marrow, but also from umbilical cord blood, adipose tissue, and other sources. However, MSCs can be difficult to expand. A therapeutic dose can require 100 million to 1.2 billion cells, yet MSC densities upon harvest can vary from 20,000 cells/cm
2
to 100,000 cells/cm
2
depending on source, culture medium, and application.
Sherman demonstrated use of Corning HYPERStack culture vessels to stream...
Although
Escherichia coli
often enables dependable, highly productive expression of nonglycosylated recombinant proteins, the efficiency with which it secretes a target protein into culture supernatant can depend greatly on that molecule’s physicochemical properties. Some proteins remain trapped in periplasm, thus diminishing process yield and productivity. In June 2021, Marcel Thoen (head of Wacker Biotech’s Global Competence Center for Cell Line Development) described how his company’s improved ESETEC (
E. coli
secretion technology) solutions can address productivity challenges raised by difficult-to-secrete recombinant proteins.
Thoen’s Presentation
Thoen emphasized that improving
E. coli
’s periplasmic-secretion efficiency raises clear advantages for upstream processing. Doing so can increase yields significantly, especially when compared with titers achieved with conventional microbial systems that generate soluble proteins. Enhancing a host’s secretion efficiency also could eliminate the need fo...
Biologics undergo extensive characterization to demonstrate their safety, purity, and efficacy. Jennifer Lawson (product manager for cell line, media, and testing solutions at Sartorius) highlighted the role of potency assays in that process. Because they reflect the complexity of biological systems, scientists must develop robust assays that will provide sufficient data for good-practice (GxP) applications. Lawson pointed out milestones in the bioassay life cycle and explored ways to help ensure method suitability.
Lawson’s Presentation
Development Criteria:
Potency assay development requires consideration of several factors in addition to typical bioassay parameters. A potency assay must be functionally relevant to a product’s mechanism of action (MoA) — balanced against the inherent variability of bioassays. Moreover, assays with multiple steps, primary cell types, and/or complex reagents are likely to hinder routine, GxP-compliant testing.
Lawson noted that cells are key sources of assay variation. U...
Regulators require testing of drug products for process-related impurities throughout development to monitor product safety, purity, and efficacy. Low levels of most impurities can be inconsequential, but patient safety demands that host-cell proteins (HCPs) be eliminated or reduced to the lowest levels practical. Enzyme-linked immunosorbent assays (ELISAs) represent a key tool in that endeavor. Antibody-coverage analysis is one part of assessing a platform kit or custom HCP ELISA. In a 15 June 2021 webinar, Jared Isaac (senior scientist at Cygnus Technologies) discussed orthogonal antibody affinity extraction (AAE) and mass spectrometry (MS) methods for that purpose. He included case studies from several cell-based processes.
Isaac’s Presentation
Viral-vector and vaccine manufacturing requires rigorous analytics for process-related impurities such as HCPs and DNA, growth-media components, enzymes, and purification process additives. HCPs can remain after multiple purification steps and must be reduced or...
For months, international partners have pressured the United States about intellectual property (IP) concerns and access to SARS-CoV-2 vaccines. As the country emerges from the worst of the pandemic, President Joe Biden now has an opportunity to shift his administration’s focus to realizing its longstanding promise of making the United States “the arsenal of vaccines for the world” (
1
). However, the country cannot accomplish that goal just by exporting mRNA vaccines and waiving IP protections. The current generation of vaccines simply cannot be produced, transported, and administered at the scale needed to tackle the COVID pandemic.
In late spring, President Biden announced that the government would begin exporting doses from its national stockpile to countries in need, starting with 20 million doses of the three vaccines that have received emergency use authorization from the Food and Drug Administration (FDA) (
2
). That announcement followed his decision to side with leaders in South Africa, India, a...
To facilitate and speed up process- and media development, online measurement techniques for shake flasks accompany manual sampling for maximum information output per cultivation. Off-gas analysis gives oxygen transfer rate (OTR), carbon dioxide transfer rate (CTR) and respiratory quotient (RQ) as quantitative measures of the physiological state of the culture. On shake flask scale, multiple cultivations are usually run in parallel. Off-gas analysis should therefore be cost effective, easy to handle and versatile to match various applications. Therefore, we developed a shake flask off-gas analysis system for non-invasive online determination of OTR, CTR and RQ. TOM (Transfer rate Online Measurement) is built modular for off-gas analysis in 4, 8, 12 or 16 individual shake flasks. The new Kuhner TOM can be applied to various shake flask sizes and types (baffled, plastic, glass) enabling the user to get information about their existing cultivation procedures.
During a May 2021 “Ask the Expert” presentation, Jigisha Patel (vice president of global regulatory compliance and technical services at Spectrum Chemical Manufacturing Corporation) emphasized the need to minimize supply-chain contingencies that lead to variability across raw materials used in biopharmaceutical manufacturing. Deviations in raw-material specifications can jeopardize good manufacturing practice (GMP) compliance and reduce drug-product efficacy and stability. Patel explained how her company’s quality management system ensures that bioprocess materials will meet specifications and process needs.
Patel’s Presentation
Patel provided three examples of supply-chain uncertainties that cause production problems. In one case, a manufacturer of a parenteral drug product purchased dry-powder sodium phosphate, but the product that arrived was more granular than expected. It clumped easily and posed risks for the company’s mixing vessels. The user’s and supplier’s quality assurance (QA) teams found that...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.