September 2022
Just coming off our big anniversary issue, we editors are still in a taking-stock mindset. That normally goes hand-in-hand with planning for the next publication year — which we can’t believe we’re doing already — but it feels more acute this time. And our 20th year has coincided with some pretty major current events, as the world begins to emerge from a pandemic amid a great deal of associated economic and sociopolitical turmoil. The effects of it all on the biopharmaceutical industry have yet to settle into something that can be qualified, much less quantified. It was a lot easier to look back at the past 20 years in our special issue than even to try imagining what the future will bring.
But unless you’re a very special particle in a very esoteric quantum-physics experiment, the stream of time moves in only one direction and carries us all along with it. Change is our inevitable companion on the journey, and on this page in the next few issues, we’ll be introducing in detail some new people, processes,...
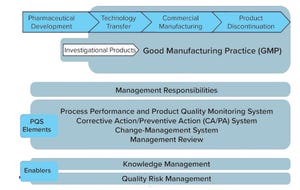
Risk, knowledge, uncertainty, decision-making: They are among the building blocks at the center of the biopharmaceutical industry that guide the daily operations of biopharmaceutical organizations, from the work of scientists during discovery to technicians manufacturing each batch. Indeed, the biopharmaceutical industry is a knowledge-based industry in which organizations satisfy patient needs while gaining competitive advantage by their ability to grow, transfer, and apply knowledge rapidly and effectively.
Many people who read the word
knowledge
have a certain implicit interpretation of the term such as
explicit knowledge
in the form of process data, methods, and specifications. But many synonyms used throughout regulatory and industry guidance alike invoke the concept of knowledge. Terms include
prior knowledge, scientific knowledge, science, product
and/or
process knowledge, experience, product development history, expertise, know-how, product and/or process understanding, lessons learned
, an...
Wetware is the focus here in Part 2.
The past couple of decades have witnessed significant advances in upstream bioprocess technologies and approaches. Since its establishment, BPI has been a facilitator of discussion both in print and at professional conferences, as well as in webcasts and news online. To mark the 20th anniversary of the publication, we surveyed articles published over the past two decades and found hundreds that highlight significant advances in both emerging and established themes in biopharmaceutical production:
• “hardware” technology (e.g., analytical instrumentation, bioreactors, and facilities) — Part 1 (
1
)
• “software” technology and knowledge (e.g., process controls, quality by design (QbD), and process analytical technology (PATs)) — Part 1
• “wetware” technology (e.g., expression systems, cell-line development, culture media, and emerging modalities such as cell and gene therapies) — Part 2.
Note that the references listed as Further Reading are but a small sampling of the h...
Allogeneic products are an attractive option for cell-therapy developers because multiple batches can be manufactured using apheresis material collected from one healthy donor — and because the resulting therapies could be made available as off-the-shelf products. The appeal of this approach is apparent from growth in allogeneic-therapy development. According to the Alliance for Regenerative Medicine, the number of clinical trials for allogeneic cell-based cancer treatments has increased by 30% over the past five years. Early in 2022, allogeneic candidates accounted for 27% of all cell-based immunotherapies under clinical evaluation (
1
).
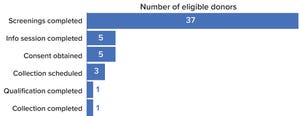
However, building a stable, reliable supply chain for cellular starting material is complex. Many processes must occur before donor material can be shipped to a biomanufacturer. Each step raises obstacles for developers, and such difficulties evolve — for both drug developers and suppliers of raw and starting materials — as a therapy moves through clinical trials and in...
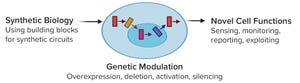
Figure 1:
Synthetic biology opens up novel cell functions by means of genetic modulation (FIGURE GENERATED USING BIORENDER SOFTWARE).
Biopharmaceuticals are produced mainly by Chinese hamster ovary (CHO) cell lines, for which advances in protein formats, bioprocesses, and bioprocess control are introducing novel challenges (
1
). Thus far, those challenges have been tackled either by technical innovations and media optimization or by advances in host-cell engineering (
2, 3
). Some technical innovations bring further challenges, such as those related to the compatibility of CHO cultures with highly automated bioprocesses and continuous high-density culture modes (
4
). With regard to host-cell engineering, most optimization efforts have been based on rational, one-dimensional approaches focusing on conventional single-gene overexpression, knockout, or knockdown (
2
). Synthetic biology offers a spectrum of molecular biology methods for redesigning organisms to useful purposes by engineering them to have ...
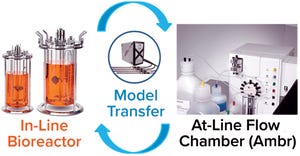
Figure 1:
Model transfer from Ambr 250 high-throughput systems (Sartorius Stedim Biotech) to and from larger-scale, stirred-tank bioreactors.
Spectroscopic sensors are powerful tools for bioprocess monitoring within the process analytical technology (PAT) initiative of the US Food and Drug Administration (FDA). The PAT framework includes process understanding based on scientific background with the aim of monitoring and controlling critical process parameters (CPPs) that influence critical quality attributes (CQAs) of final biological products. The driving force for PAT implementation is a need to realize consistent product quality, process intensification, and real-time manufacturing control (
1, 2
).
Using real-time spectroscopic measurements of bioprocesses allows for monitoring of CPPs such as biological, chemical, and physical variables throughout biotechnological production. Raman spectroscopy has become a first-choice PAT for monitoring and controlling upstream production processes (
3
). The tech...
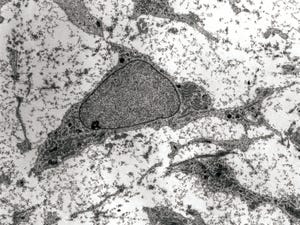
Transmission electron micrograph of a mesenchymal stromal cell
ROBERT M. HUNT (
HTTPS://EN.WIKIPEDIA.ORG
)
Mesenchymal stromal cells (MSCs) are multipotent, self-renewing progenitor cells that can differentiate into adipocytes, chondrocytes, and osteocytes (
1
). Cultured MSCs are plastic-adherent and spindle-shaped, and they express cell-surface markers CD44, CD73, CD90, and CD105, but not CD14, CD34, CD45, CD11b, CD79a, CD19, or HLA-DR (
2, 3
). First isolated from bone marrow (BM), human MSCs have been investigated extensively in clinical studies. MSCs also have been isolated from adipose tissue (
4
) and peripheral blood (
5
). Perinatal organs and tissues such as amniotic membrane, placenta, and umbilical cord (UC) also have been shown to be rich sources of MSCs (
6
). The UC consists of a cord lining (CL), Wharton’s jelly (WJ), and a perivascular region, all of which have been documented as sources for isolation and expansion of MSCs (
7–9
). WJ reportedly yields 10,000 to 4.7 million MSCs per cent...
Cathepsins and other proteases derived from host cells can cleave therapeutic proteins, diminishing product efficacy.
https://www.wikipedia.com
Biopharmaceuticals are produced in genetically modified cells; thus, they contain process-and product-related impurities. Those deriving from manufacturing processes include host cell DNA/RNA, viral DNA/RNA, cellular debris, lipids, and host-cell proteins (HCPs) (
1
). Mammalian, bacterial, fungal, insect, and plant cell lines have been used to overexpress recombinant proteins. Currently, the most frequently used hosts for biomolecule synthesis are
Escherichia coli
and Chinese hamster ovary (CHO) cells.
E. coli
has been used to produce heterologous proteins since the beginning of the biotechnology industry. The expression system is attractive because it grows quickly and effectively using inexpensive culture media. It also produces large amounts of protein from high-copy plasmids. Although heterologous proteins expressed in this species are not glycosylated, ...
The use of approved advanced therapy medicinal products (ATMPs) remains limited despite their potential to address unmet medical needs. One example uses chimeric antigen receptor (CAR) T cells for treatment of refractory lymphoma (
1
). Typically, such medicinal products begin with cells that are harvested from a patient and genetically programmed to recognize and eliminate tumor cells upon reinfusion. Several cell therapies based on this and other technologies are approved for use in the United States, Europe, and China (
2
). Given their indications, up to 60,000 patients could be treated in those markets annually. However, in 2018 and 2019 only about 1,750 patients received CAR-T treatments, and eligible recipients faced long waiting lists with manufacturers (
3
). Autologous use of a patient’s own blood cells has significant consequences on the manufacturing and quality control (QC) of such therapies. In essence, one product for one patient represents one batch — and as a result, the methods establish...
An estimated 300 million people worldwide live with rare diseases, and over 70% of such disorders are caused by genetic mutations (
1, 2
). Cell and gene therapies offer hope and potential cures for many previously untreatable diseases. Accordingly, the global gene therapy market is expected to be worth USD 5.02 billion by 2028, a significant growth from USD 1.46 billion in 2020 (
3
).
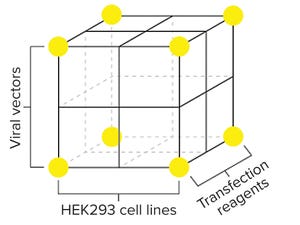
Manufacturing gene therapies will be a key challenge over the next two decades. But just a few decades ago, the industry was in a similar position for recombinant protein production. The lessons learned from generating high-performing Chinese hamster ovary (CHO) cell lines can be used to accelerate progress toward a gene therapy process platform.
Viral vectors are ideal vehicles for gene transfer because of their high transduction efficiency, effective gene delivery, and stable gene expression. Immortalized human embryonic kidney (HEK) 293 cells are the optimal host cells for virus production. Together, HEK293 cells and vir...
In honor of BPI’s 20th anniversary as a publication, the editors invited authors to reflect on the industry’s past, present, and future. Below, the chief operating officer of Selexis offers her perspectives along with responses to questions from the editorial team.
Significant Scientific and Technological Innovations
One of the most important innovations in bioprocessing has been the combination of next-generation sequencing with bioinformatics. The ability to sequence a host cell-line genome and transcriptome rapidly and cost-effectively and the development of bioinformatics to analyze those data have enabled significant advances in cell-line development (CLD).
Analyses of host manufacturing cell lines enables scientists to determine where potential production issues might occur. Secretory requirements can differ from protein to protein, so genomic and/or transcriptomic analyses of parent manufacturing cell lines can help determine where deficiencies or inappropriate expression of cellular proteins could...
Membrane adsorbers can be a simple and effective choice for anion-exchange (AEX) purification of biopharmaceuticals. However, as Sherri Dolan (global technology consultant for virus clearance at Sartorius) explained during a May 2022 presentation, biomanufacturers generally do not leverage their membranes’ full loading capacities. Doing so could improve process economics and decrease costs for several downstream applications.
Dolan’s Presentation
Membrane adsorbers are ideal for flow-through AEX applications (e.g., secondary purification and polishing) because they can be used at high flow rates and loaded to high capacities (in flow-through mode). Biomanufacturers often apply adsorbers only for small-scale activities, the rationale being that scale-up necessitates development of a costly membrane configuration. However, membrane adsorbers often are not loaded to their full capacities. Doing so could improve process economics and enable application of this technology at larger scales.
Dolan described her ...
During a May 2022 Ask the Expert presentation, Ivana Petrović Koshmak (head of upstream process development at BIA Separations, part of Sartorius) highlighted the gene- therapy industry’s need for fast, reliable analytics that work with both up- and downstream samples. High-performance liquid chromatography (HPLC) methods can fulfill that need, but process development (PD) scientists would need to account for the complexity of bioreactor samples to make such methods feasible for end-to-end analytics. Koshmak described a novel instrument that is designed to characterize adenoassociated virus (AAV)-vector quality attributes from transfection through fill–finish.
Koshmak’s Presentation
Koshmak emphasized the advantages of monolith chromatography columns for AAV process analytics. In bead-based media, diffusion limits mass transfer. Thus, samples take a long time to process. Beads also provide little capacity for large biomolecules and generate countercurrent flow, causing shear stress that diminishes product...
High-performance liquid chromatography (HPLC) has become a leading analytical method for biopharmaceutical process development and optimization, particularly for therapies that leverage plasmid DNA (pDNA), messenger RNA (mRNA), and viral vectors. In July 2022, Blaž Goričar (manager of process analytics development at BIA Separations, a Sartorius company) demonstrated the features of his company’s PATfix software for HPLC data processing and analytics. He described how the program can simplify method execution and enhance evaluation of resulting data.
Photo 1:
PATfix software from BIA Separations, a Sartorius company.
Goričar’s Presentation
PATfix software is a client–server application for simple, secure access to HPLC data. A small personal computer is used as controller to transmit process data automatically from connected chromatography equipment (e.g., pumps, detectors, and autosamplers) to a server for processing, storage, and analysis. Desktops, laptops, and other clients can access collected data ...
Analytical groups are developing more methods than ever to address mounting demand for biopharmaceuticals. Still, such teams need to work within tight timelines to help candidate therapies advance quickly through clinical trials. During a May 2022 presentation, Rajgopal Rudrarapu (senior scientist at the Almac Group) pointed out that regulatory agencies allow biomanufacturers to apply prior knowledge to facilitate analytical development and validation. He described how his company leveraged prior knowledge to develop and validate a capillary isoelectric focusing (cIEF) method for analysis of monoclonal antibody (MAb) products.
Rudrarapu’s Presentation
Several guidelines from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) encourage use of established scientific principles, peer-reviewed literature, and documented manufacturing experiences to support analytical development. Thus, when Almac sought to install new software on its PA 800 Plus capil...
Regulatory agencies are scrutinizing gene-therapy product quality more closely than ever, yet such therapies still are produced in small batches and at high costs. Thus, drug companies are struggling to make safe and efficacious gene therapies available to patients. In an April 2022 presentation, Tim Kelly (chief executive officer of Oxford Biomedica Solutions, OXB Solutions) emphasized the importance of addressing both process output and product quality when manufacturing adenoassociated virus (AAV)-based gene therapies. Such an approach requires deep expertise and comprehensive capabilities.
Kelly’s Presentation
Product quality has become a priority for the gene-therapy industry amid clinical-trial holds relating to problems with chemistry, manufacturing, and controls (CMC). Meanwhile, regulatory requirements are increasing for AAV-based products. Authorities expect companies to know product critical quality attributes (CQAs) at increasingly early clinical stages. Agencies also are requesting data from ...
Conventional ultraviolet–visible spectroscopy (UV-vis) instruments use fixed optical pathlengths to measure analyte concentrations in biological samples. As Paul Mania (bioanalytics application specialist at Repligen) pointed out during an April 2022 presentation, such an approach requires sample dilution, which introduces risks for error and increases operator workload. Mania explained how the CTech SoloVPE instrument measures analyte concentrations at line without need for dilution, simplifying and accelerating analysis of samples from several downstream operations.
Mania’s Presentation
Traditional UV-vis instruments measure analyte absorbance one time across a fixed pathlength, then use that value to determine analyte concentration. But absorbance values from biological samples exceed the linear range of most such systems. Thus, samples must be diluted. That complicates concentration measurement, Mania explained. Operators need to estimate an analyte’s concentration, perform volumetric or gravimetric d...
Manufacturing costs remain high for gene therapies delivered by adenoassociated virus (AAV) vectors. The biopharmaceutical industry must minimize such expenses because they account for significant proportions of the high prices that patients pay for treatment. During a June 2022 webinar, Emmanuelle Cameau (leader for cell and gene therapy strategic technology partnerships at Pall Corporation) joined Maxime Dumont (cell and gene therapy product manager at webcast sponsor Polyplus-transfection) to describe their companies’ efforts to model AAV manufacturing costs. Cameau and Dumont focused on productivity improvements that can be achieved by optimizing transfection reagents.
The Presentations
Dumont explained that capital expenditure (CapEx) and operational expenses (OpEx) represent 60–70% and 30–40% of AAV manufacturing costs, respectively. Among OpEx, upstream labor and materials account for a larger proportion of cost of goods sold (CoGS) than do downstream materials, with plasmids incurring the highest ...
Although culture-media optimization accounts for a relatively small part of process development, selections made at that stage strongly influence overall bioprocess productivity. Yaron Silberberg (chief scientist at the Ajinomoto Genexine CELLiST Solution Center) joined BPI in July 2022 to present strategies for enhancing media performance. He focused on how emerging tools for statistical analysis and process control facilitate media development for cultures based on Chinese hamster ovary (CHO) cell lines.
Silberberg’s Presentation
Because materials applied during cell-line development are not optimized for large-scale protein production, host cells usually undergo sequential adaptation to a medium suitable for industrial applications. Batch and/or fed-batch cultures in shake flasks or microbioreactors are used to screen potential feed regimens. Then, optimization activities are conducted to assess the effects of process parameters and supplements on expression titers and batch productivity. Advanced opti...
Photo 1:
The Samux
MP
system for mass photometric analysis of adenoassociated virus (AAV) vectors for gene therapies.
Current production processes for gene therapies based on adenoassociated virus (AAV) vectors generate many empty capsids. That problem complicates vector purification and diminishes product safety and quality. In a June 2022 webinar, Gareth Rogers (product manager at Refeyn Ltd.) observed that developers could benefit significantly from analytical instruments that assess empty-to-full (E:F) capsid ratios rapidly. He explained how the Samux
MP
mass photometry system (Photo 1) could address such needs. Kirsty McManus (senior scientist in AAV characterization at Pharmaron Gene Therapy) described how her company, a contract development and manufacturing organization (CDMO), has used the instrument to facilitate optimization during downstream process development.
Rogers’s Presentation
Rogers highlighted several difficulties associated with the large number of empty capsids produced during AA...
Biopharmaceutical companies continue to invest significantly in technologies that support process intensification (PI), including equipment for high-density and perfusion-mode cell cultures. During a July 2022 Ask the Expert webinar, Yuliya Mikhed (product manager for Biostat RM bioreactors at Sartorius) highlighted drivers for PI solutions, then described how an intensified modular seed train could help users to increase cell culture productivity and flexibility while reducing cost of goods (CoG).
Mikhed’s Presentation
Sartorius customers have identified several drug development “pain points.” Biologics developers are under pressure to shorten commercialization timelines for new therapies. However, mounting regulatory expectations are compelling companies to increase yields and generate enough material for testing. Companies such as small contract manufacturing organizations (CMOs) need flexible equipment to enable multiproduct manufacturing and to reduce turnover time between programs. And scale-up to c...
Bioprocessing materials such as cell-culture media, sera, and reagents are used in the development of different drug products such as recombinant proteins, immunotherapies, and regenerative medicine. Biopharmaceutical research materials include DNA/RNA isolation reagents, chemicals, buffers, stains, and washing solutions.
Media provide nutrients essential for cell growth in culture. Advancements in culture media (both serum-based and serum-free) have enabled researchers to culture different types of cells and microorganisms, including mammalian and bacterial host cells for protein expression (e.g., Chinese hamster ovary (CHO) cells and
Escherichia coli
). That work has helped improve understanding of cell biology. Cell culture requires the preparation of solids, semisolids, or liquids for the growth, storage, maintenance, and transport of microorganisms and other cell types and cell lines. Chemically defined basal media also are used to support optimal cell growth.
Sera such as fetal bovine serum (FBS) h...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.