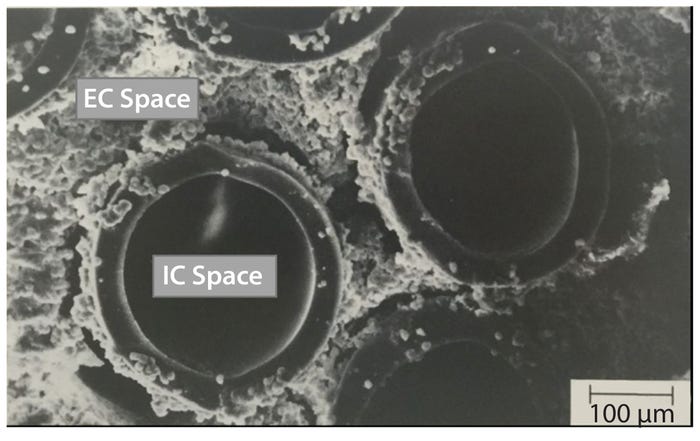
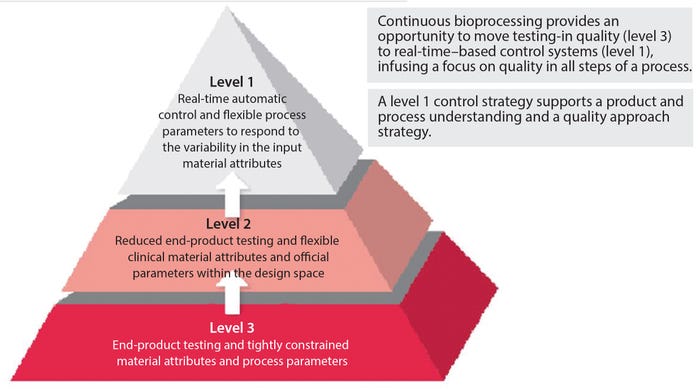
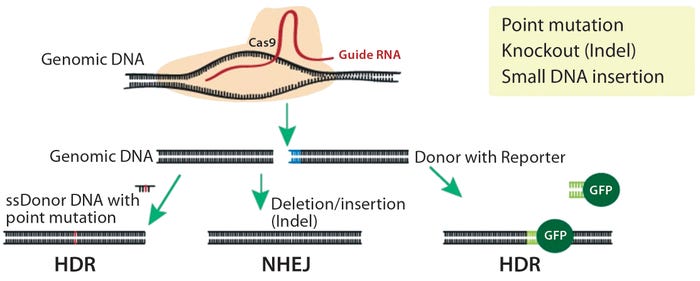
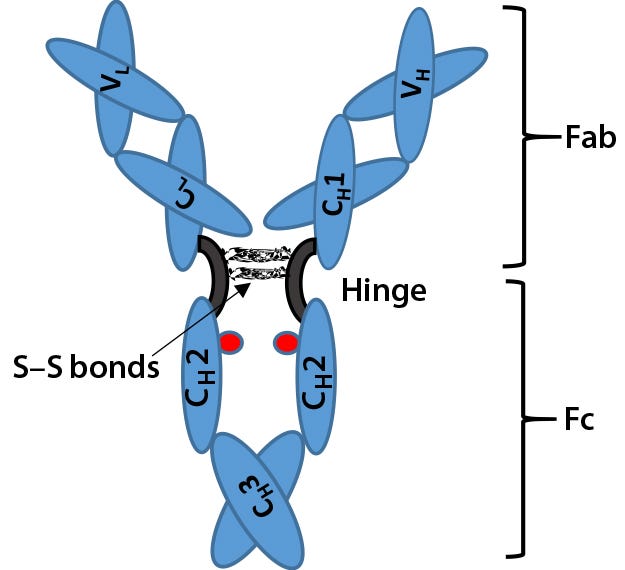
Voices of Biotech
Podcast: Sustainability is about health equity, says ScaleReady and Germfree
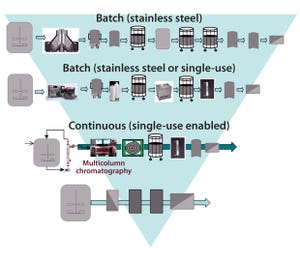
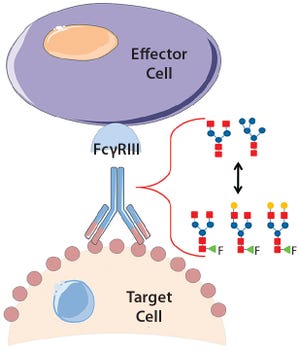
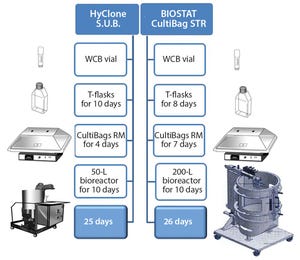
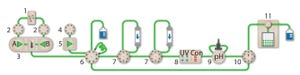
ScaleReady and Germfree discuss the need to rethink sustainability and move towards a more standardized and simplistic manufacturing model to ensure health equity can be achieved.